INTRODUCTION
Participant enrollment in clinical trials is challenged by a multitude of structural-, clinical-, physician-, and individual-level barriers to participation.1 In addition to slow clinical trial accrual, there is often under-representation of racial/ethnic minorities who encounter even greater barriers to participation.2 , 3 The coronavirus disease 2019 (COVID-19) pandemic has further challenged participant enrollment.4 Nationally representative data on attitudes toward clinical trials are limited. This cross-sectional study aims to describe perceived knowledge, trusted informational sources, and attitudes about clinical trial participation overall and by race/ethnicity in the U.S.
METHODS
The Health Information National Trend Survey 5, Cycle 4 was conducted between February 24, 2020 and June 15, 2020 by the National Cancer Institute. This is a mailed complex survey of U.S. civilian, non-institutionalized adults aged ≥18 years (response rate=37%).5 Respondents completed a self-administered mail questionnaire on demographic and health-related information. This analysis of publicly available data was deemed exempt from review by the Johns Hopkins University School of Medicine IRB.
Descriptive statistics were used to describe perceived knowledge and trusted informational sources about clinical trials and perceived factors that would influence clinical trial participation overall and by race/ethnicity. Rao–Scott chi-square tests were used to assess differences by race/ethnicity. Analyses accounted for the complex survey design and incorporated jackknife replicate sampling weights using Stata/MP, version 15.2. Except for sample sizes, all reported data were weighted.
RESULTS
There were 3,865 respondents. The mean age was 48 (SD=18.1) years; 47.6% (n=1,561) were male, 50.2% (n=2,204) were female, and 2.2% (n=100) did not report their sex. A total of 58.7% (n=2,133) were non-Hispanic White, 10.3% (n=481) were non-Hispanic Black, 15.7% (n=596) were Hispanic, 7.9% (n=280) were of other race/multiracial, and 7.3% (n=375) did not report their race/ethnicity.
Only 291 (7.0%) respondents had ever heard of ClinicalTrials.gov. There were 1,406 (41.3%) respondents who reported knowing nothing about clinical trials. The proportion who reported knowing nothing about clinical trials significantly varied by race/ethnicity (non-Hispanic White, n=639 [36.8%]; non-Hispanic Black, n=182 [40.8%]; Hispanic, n=301 [51.9%]; other race/multiracial, n=105 [53.6%]; unknown race/ethnicity, n=179 [48.9%]; p=0.003).
The majority reported that their most trusted informational source on clinical trials was their healthcare provider (n=2,686 [73.3%]); others also endorsed health organizations/groups (n=481 [13.5%]), government health agencies (n=196 [5.9%]), patient support groups (n=163 [4.1%]), family and friends (n=85 [2.9%]), and drug companies (n=16 [0.3%]). Healthcare providers were the most trusted source among each race/ethnicity group (non-Hispanic White, n=1,525 [74.6%]; non-Hispanic Black, n=326 [75.5%]; Hispanic, n=394 [66.9%]; other race/multiracial, n=190 [68.4%]; unknown race/ethnicity, n=251 [78.9%]; p=0.087).
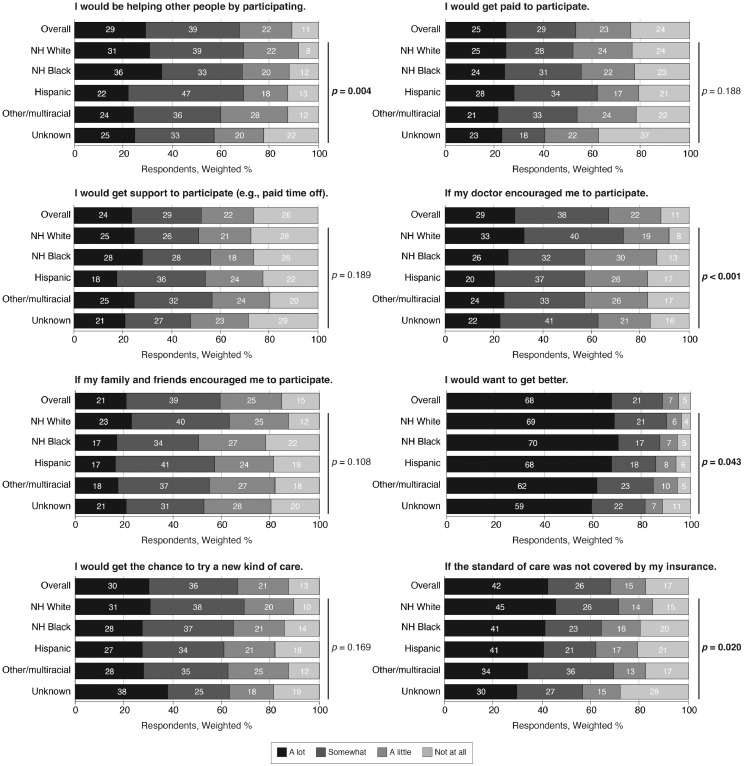
Respondents endorsed various factors that would influence their decision to participate in a clinical trial (Figure 1 ). Remarkably, 67.7% (n=2,368) of respondents reported that motivation to get better would have a lot of influence. The distribution of responses was similar by race/ethnicity for some but not all the examined factors. For instance, compared with 32.7% (n=729) of non-Hispanic White respondents who reported that their doctor's encouragement would have a lot of influence on their decision, only 25.6% (n=125) of non-Hispanic Black respondents (p=0.04) and 20.3% (n=140) of Hispanic respondents (p<0.001) reported this.
Figure 1.
Perceived factors that would influence one's decision to participate in a clinical trial.
Note: Participants were asked, Imagine that you had a health issue and you were invited to participate in a clinical trial for that issue. How much would each of the following influence your decision to participate in the clinical trial? The stacked bar charts show the frequency distribution of Likert-type scale responses. Data were calculated among available cases. Responses are displayed overall and by race/ethnicity. p-values were calculated using (second-order) design-adjusted Rao−Scott chi-square tests.
NH, non-Hispanic.
DISCUSSION
In this national survey, there was limited perceived knowledge about clinical trials, which was even lower among some racial/ethnic minority groups (e.g., Hispanics). Notably, healthcare providers were the most trusted informational source on clinical trials, regardless of race/ethnicity. These data support leveraging healthcare providers in the provision of clinical trial information; however, this alone is unlikely to sufficiently improve racial/ethnic representation. Indeed, there were important racial/ethnic differences in the degree of confidence in one's doctor to influence their decision to participate in a clinical trial. In addition, the decision to participate is complex,6 and racial/ethnic minorities face numerous systemic barriers to clinical trial participation beyond individual-level knowledge and attitudes.2 , 3 , 7 , 8
Limitations
This study may be limited by selection bias. There was a high nonresponse rate, and it is likely that respondents are more likely than nonrespondents to participate in research in general, perhaps leading to overestimates of knowledge and positive attitudes related to clinical trials. In addition, data were collected during the early phase of the COVID-19 pandemic, which may have influenced responses. Furthermore, reported attitudes may not be generalizable to all patient populations or apply to all trials with varying intensities of study requirements.
CONCLUSIONS
These data combined with decades of research underscore that concerted efforts, including improving health literacy and patient trust, are needed to ensure diversity, equity, and inclusion in clinical trials.3 , 9 , 10 Attitudes toward clinical trials should be monitored as the COVID-19 pandemic continues to progress.
Acknowledgments
ACKNOWLEDGMENTS
The funders of this study had no role in the design and conduct of the study; collection, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
This work was supported in part by the U.S. Department of Defense Peer-Reviewed Medical Research Program (W81XWH1810742 to AART); the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID); and extramural support from NIAID (R01AI120938 and R01AI128779 to AART and T32AI102623 to EUP).
No financial disclosures were reported by the authors of this paper.
CRediT Author Statement
Eshan U. Patel: Conceptualization, Formal analysis, Visualization, Writing - original draft. Xianming Zhu: Formal analysis, Writing - original draft. Thomas C. Quinn: Funding acquisition, Writing - review & editing. Aaron A.R. Tobian: Funding acquisition, Supervision, Writing - review & editing.
REFERENCES
- 1.Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111(3):245–255. doi: 10.1093/jnci/djy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16–e31. doi: 10.2105/AJPH.2013.301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Academy of Sciences Engineering and Medicine . The National Academies Press; Washington, DC: 2016. Strategies for Ensuring Diversity, Inclusion, and Meaningful Participation in Clinical Trials: Proceedings of a Workshop. [DOI] [PubMed] [Google Scholar]
- 4.Unger JM, Blanke CD, LeBlanc M, Hershman DL. Association of the coronavirus disease 2019 (COVID-19) outbreak with enrollment in cancer clinical trials. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finney Rutten LJ, Blake KD, Skolnick VG, Davis T, Moser RP, Hesse BW. Data resource profile: the National Cancer Institute's Health Information National Trends Survey (HINTS) Int J Epidemiol. 2020;49(1) doi: 10.1093/ije/dyz083. 17–17j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houghton C, Dowling M, Meskell P, et al. Factors that impact on recruitment to randomised trials in health care: a qualitative evidence synthesis. Cochrane Database Syst Rev. 2020;10(10) doi: 10.1002/14651858.MR000045.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bass SB, D'Avanzo P, Alhajji M, et al. Exploring the engagement of racial and ethnic minorities in HIV treatment and vaccine clinical trials: a scoping review of literature and implications for future research. AIDS Patient Care STDs. 2020;34(9):399–416. doi: 10.1089/apc.2020.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unger JM, Hershman DL, Till C, et al. “When offered to participate”: a systematic review and meta-analysis of patient agreement to participate in cancer clinical trials. J Natl Cancer Inst. 2021;113(3):244–257. doi: 10.1093/jnci/djaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heller C, Balls-Berry JE, Nery JD, et al. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: a systematic review. Contemp Clin Trials. 2014;39(2):169–182. doi: 10.1016/j.cct.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark LT, Watkins L, Piña IL, et al. Increasing diversity in clinical trials: overcoming critical barriers [published correction appears in Curr Probl Cardiol. 2021;46(3):100647] Curr Probl Cardiol. 2019;44(5):148–172. doi: 10.1016/j.cpcardiol.2018.11.002. [DOI] [PubMed] [Google Scholar]