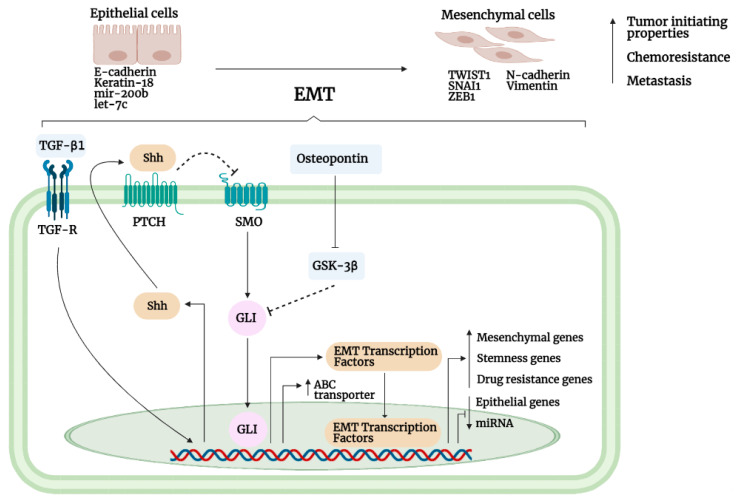
Figure 5.
A schematic representation of canonical and noncanonical GLI regulation in promoting EMT and chemoresistance. TGF-β1 stimulation induced the transcriptional upregulation of Shh, which activates canonical Hh-GLI signaling in an autocrine manner. On the other hand, OPN inactivates GSK3β, promoting the activation of GLI independent of canonical input. Activated GLI translocates into the nucleus, where it upregulates the expression of EMT transcription factors (e.g., TWIST1, SNAI1, ZEB1). In turn, upregulated EMT transcription factors induce the expression of mesenchymal (e.g., N-cadherin, Vimentin), stemness (e.g., SOX2, OCT4, ALDH), and drug resistance genes (e.g., ALDH, ABC transporters) while concomitantly downregulating epithelial (E-cadherin) genes and miRNAs (mir-200b and let-7 family). Consequently, this results in the formation of mesenchymal cells with tumor-initiating-like properties and the increased capability to metastasize and resist cytotoxic chemotherapeutics. Additionally, increased GLI1 activity also upregulates ABC transporters, further enhancing chemoresistance by increasing drug efflux in mesenchymal cells.