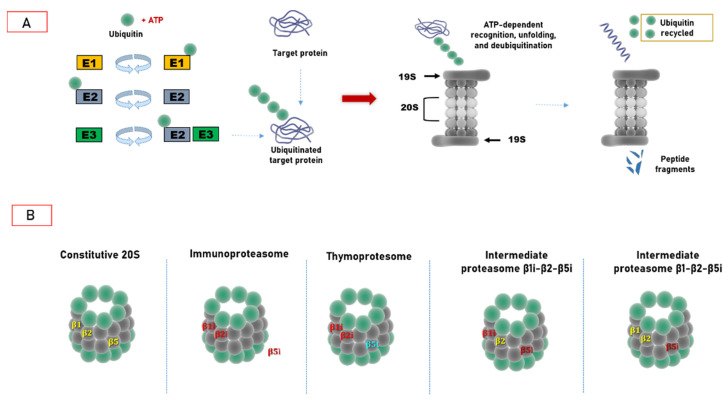
Figure 1.
Ubiquitin–proteasome system. (A) General UPS organization. The Ub target protein conjugation cascade consists of three classes of ubiquitin ligases: E1, E2, and E3. Ub is activated by E1, forming a high-energy thioester bond; then, activated Ub is transferred to E2, and then E3 attaches Ub to a specific polypeptide substrate. At the end stage, the substrates are recognized and subjected to ATP-dependent degradation by the 26S proteasome. (B) Structural heterogeneity of the proteasome core particle; 20S constitutive proteasome contains the standard catalytic subunits β1 (caspase-like activity), β2 (trypsin-like activity), and β5 (chymotrypsin-like activity). The immunoproteasome incorporates the inducible catalytic subunits β1i, β2i, and β5i, which have different catalytic specificities. The thymoproteasome is expressed only by cortical thymic epithelial cells and is characterized by the presence of β1i-β2i, and β5t subunits. Finally, intermediate proteasomes contain a mix of constitutive and inducible subunits: β1i,-β2-β5i and β1-β2, and β5i, respectively.