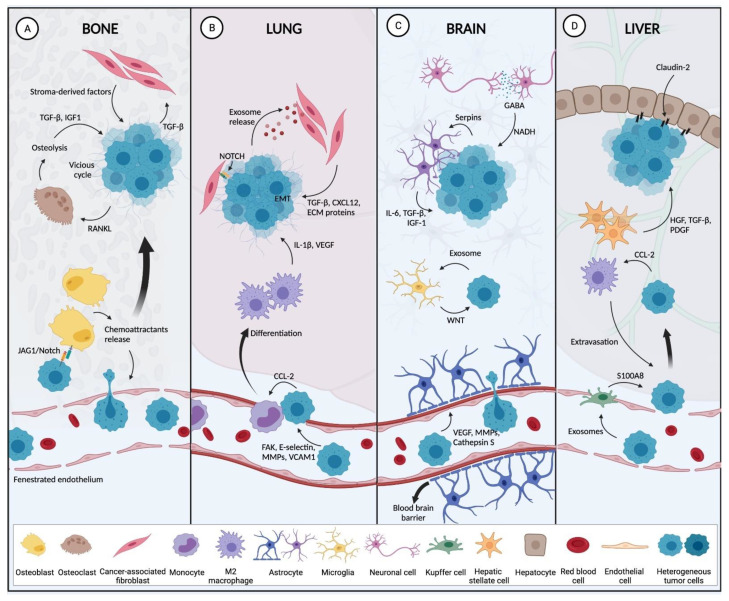
Figure 2.
Host stromal cells at the metastatic site promote the establishment of the breast tumor. (A) In the bone microenvironment, chemoattractant released by osteoblasts recruit circulating tumor cells (CTCs) from the circulation to extravasate into the bone stroma. Once in the bone environment, interactions between BC cells and osteoblasts occur via JAG1/Notch and CAFs (through TGF-β), facilitating bone metastasis. There, BC cells then secrete factors to promote osteolysis, resulting in the release of factors that stimulate tumor growth, and thereby generating a vicious cycle. (B) In the lung capillaries, the expression of FAK, E-Selectin, VCAM1 and MMPs are involved in tumor extravasation to the lung parenchyma. Tumor cells now have the capacity to recruit monocytes from the circulation to differentiate into M2 macrophages, which then secrete pro-metastatic factors (VEGF, IL-1β). BC cells also secrete exosomes that stimulate CAF to release cytokines, growth factors and ECM components to create a pro-tumorigenic niche. (C) Once within the confines of the brain, tumor cells produce cathepsin S, MMPs and VEGF to overcome the blood-brain barrier in order to colonize the brain. They then stimulate astrocytes to secrete IL-6, IGF-1 and TGF-β that result in tumor expansion. Exosomes are also secreted by BC cells that stimulate microglia to support metastasis through WNT signaling. BC cells also take advantage of neurotransmitters secreted by neurons as bio-precursors to generate NADH that support tumor growth in the brain. (D) To promote their extravasation into the liver stroma, BC cells secrete exosomes that stimulate Kupffer cells to produce S100A8 resulting in liver-specific metastasis. BC cells also modulate M2 macrophages which also promote tumor extravasation. Hepatic stellate cells, secrete HGF, TGF-β and, PDGF to induce liver metastasis. Interaction of BC cell enriched in claudin-2 and hepatocytes also result in liver metastasis establishment (Created with BioRender.com, accessed on 12 September 2021).