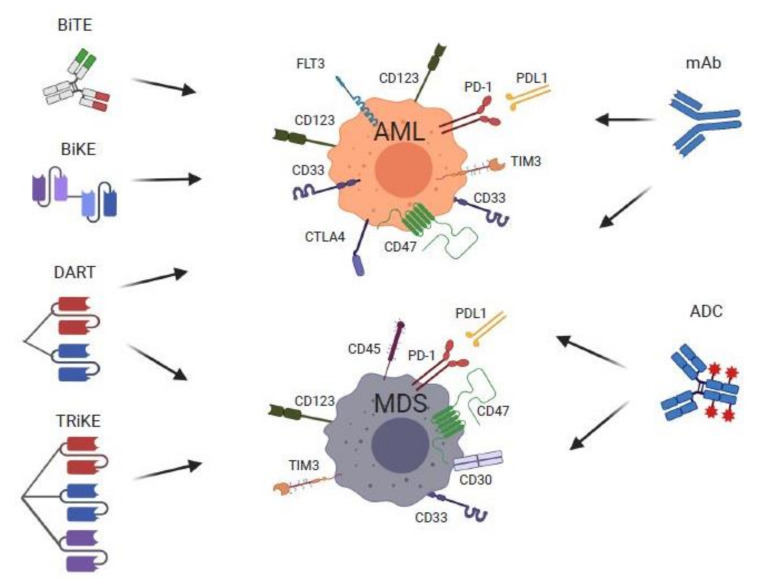
Figure 2.
Monoclonal antibodies (mAb), antibody-drug conjugates (ADC), and novel engineered monoclonal antibody therapies for MDS and AML and their immune targets (Middle panel). Emerging mAb immunotherapies include antibodies directed against immune checkpoint molecules PD-1/PD-L1, CTLA-4, and TIM3, the macrophage mediated phagocytosis inhibitor CD47, and Fc-optimized mAbs targeting CD33 and CD123. Engineered mAb therapies (Left panel) have a basic common mechanism of action by forming bonds between a tumor-associated antigen and CD3, resulting in physical interaction between T-cells and leukemic cells. These molecules include bi-specific T-cell engagers (BiTEs)—fusion of two single-chain variable fragments (scFvs) of two unique antibodies (red and green) into a single peptide chain with affinity of one to T-cells via CD3 and the other to tumor molecules, dual-affinity re-targeting molecules (DARTs)—similar to the BiTE construct with addition of a disulfide linker for increased stability, bi- and tri-specific killer cell engager (BiKEs and TriKEs)—molecules consisting of a single-chain variable fragment (scFv) fused by a heavy and variable chain, that are linked to the scFV of one (BiKE) or two (TriKE) antibodies via CD16, a receptor on NK cells that upon stimulation activate various cytokines and induce a cytolytic response by targeting CD33, CD123, and FLT-3 on tumor cells. ADCs include compounds targeting CD33, CD123, CD45, and CD30. Image created with BioRender.com.