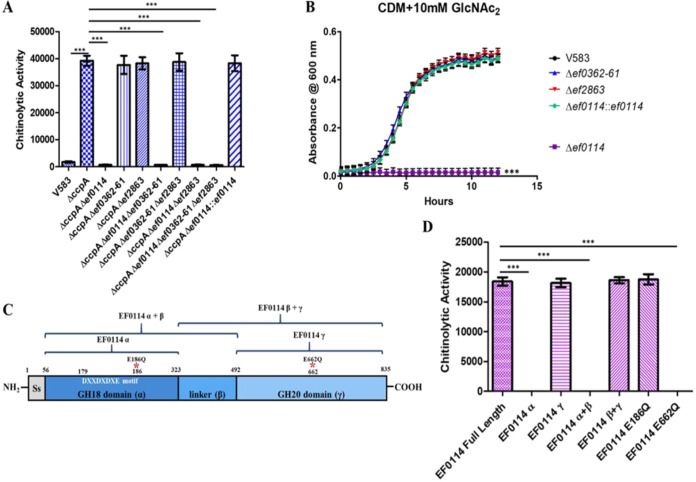
FIG 2.
(A) Chitinolytic activity from culture supernatants were measured by determining the ability to hydrolyze the fluorogenic substrate 4MU-methylumbelliferyl-β-d-N-acetylglucosamine (GlcNAc2 analogue). Chitinolytic activity is expressed as relative fluorescence emitted at 485 nm by the absorbance of the culture at OD600. Each graph shows the average from three biological replicates, with three technical replicates each time (n = 9). The statistical significance was calculated using an unpaired t test; ***, P < 0.0001. (B) Growth of E. faecalis in CDM supplemented with 10 mM chitobiose (GlcNAc2). Each graph shows the average from three biological replicates, with three technical replicates each time (n = 9). The statistical significance was calculated using a two-way ANOVA test; ***, P < 0.0001. (C) Representation of the EF0114 domain structure. The putative signal peptide sequence is located between amino acid residues 1 and 56. The GH18 catalytic domain, described as α, is located between amino acid residues 56 and 323, with the GH18 DXXDXDXE catalytic motif located between amino acid residues 179 and 186. The uncharacterized linker region is described as the β domain (between amino acid residues 323 and 492). The GH20 domain (γ) is located between amino acid residues 492 and 835. Listed above the EF0114 domain schematic are representations of the various EF0114 recombinant domain variants used in panel D. (D) Chitinolytic activity of purified recombinant EF0114 variants against the fluorogenic substrate 4MU-methylumbelliferyl-β-d-N-acetylglucosamine (GlcNAc2 analogue). Chitinolytic activity is expressed as the fluorescence emitted at 485 nm. Each graph shows the average from three biological replicates, with three technical replicates each time (n = 9). The statistical significance was calculated using an unpaired t test; ***, P < 0.0001.