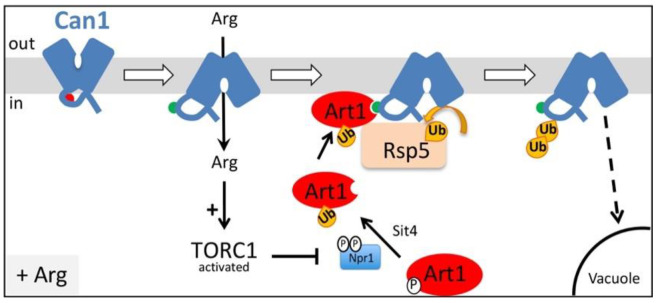
Figure 1.
Model for Art1-dependent Can1 endocytosis in response to Arg transport. In the absence of substrate, Can1 is mostly found in an outward-facing conformation and this structures the N-terminal tail in a way that masks the binding site for Art1 (aa 70–81, red hemicycle). Once Arg is added in the medium, a transient shift of the permease to an inward-facing conformation of the transport cycle repositions the N-terminal tail and exposes the binding site for Art1 (now green hemicycle). Arg uptake also stimulates TORC1, leading to inactivation of Npr1, and activation of Art1 via de-phosphorylation. Activated Art1 can then recognize the exposed binding site at the N-tail of Can1, resulting in Rsp5-mediated ubiquitylation of Can1, endocytosis and sorting to the vacuole for degradation.