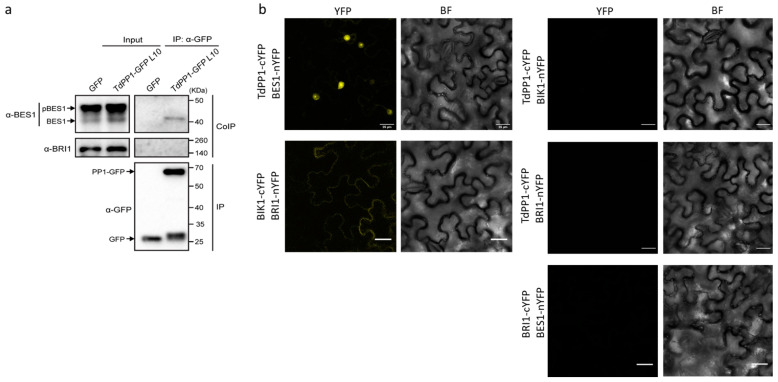
Figure 5.
TdPP1 binds to unphosphorylated BES1. (a) TdPP1 co-immunoprecipitates with BES1 but not with BRI1. Total proteins were extracted from TdPP1-GFP L10 and 35S::GFP transgenic line as negative control. The protein extracts were immunoprecipitated using anti-GFP Trap beads. Total (input), immunoprecipitated (IP) and co-immunoprecipitated (CoIP) proteins were analyzed by immunoblotting. GFP was used as a negative control for Co-IP. TdPP1-GFP and GFP were detected with anti-GFP antibody (α-GFP), BES1 with anti-BES1 (α-BES1) antibody and BRI1 with anti-BRI1 (α-BRI1). The upper arrow indicates the band of the phosphorylated BES1 (pBES1), and the lower arrow indicates the band of dephosphorylated BES1 (BES1). (b) TdPP1 interacts with BES1 and not with BRI1 in Nicotiana benthamiana. N.benthamiana leaves were co-agroinfiltrated with Agrobacterium strains expressing TdPP1 protein fused to the C-terminus half of YFP and BES1 or BRI1 proteins fused to the N-terminus half of YFP and observed with confocal microscopy. Images show YFP fluorescence in yellow (YFP) and cells in bright field (BF). The proper expression of BRI1 was confirmed by positive interaction monitored as a fluorescence signal emitted by interaction between BRI1 and BIK1. Scale bars represent 25 μm.