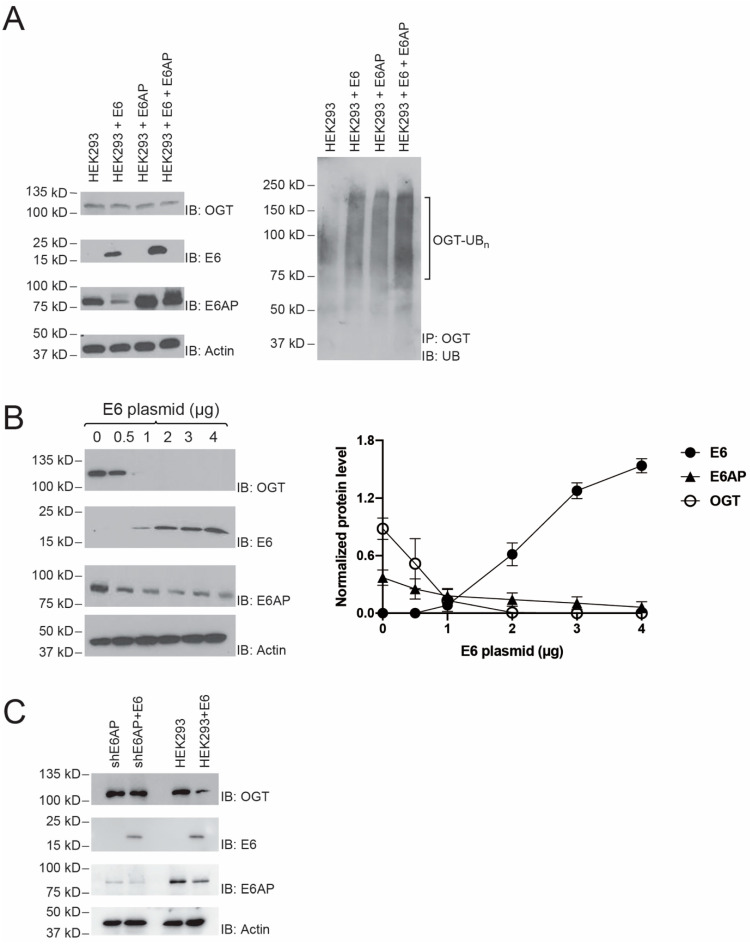
Figure 3.
E6 enhanced OGT ubiquitination and degradation mediated by E6AP in HEK293 cells. (A) E6 enhanced OGT ubiquitination in the cells. HEK293 cells were transfected with plvx-E6AP or pLenti-E6 plasmids separately or together, and the expression of E6 and E6AP was confirmed by immunoblotting with an anti-E6 antibody or an anti-E6AP antibody (left panel). After 4 h treatment of MG132, ubiquitination of OGT was compared among blank HEK293 cells (HEK293), HEK293 cells expressing recombinant E6 (HEK293 + E6), HEK293 cells expressing recombinant E6AP (HEK293 + E6AP), and HEK293 cells expressing both recombinant E6AP and E6 (HEK293 + E6AP + E6) (right panel). OGT ubiquitination enhanced by E6 was confirmed by the elevated ubiquitination levels of OGT in cells with co-expression of E6AP and E6. (B) E6 decreased the steady-state level of OGT in HEK293 cells. Cells were transfected with different amounts of E6 plasmid. Levels of OGT were assayed by immunoblotting of the cell lysate probed with an anti-OGT antibody. Quantitative analysis of OGT level in correlation with the amount of E6 expression was plotted in the panel on the right. Data points show mean ± S.E. of three or more experiments. The vertical bars in (B) represent SEM from three independent experiments (n = 3). (C) The dependence on E6AP for regulating OGT level in the cells. shE6AP and HEK293 cells were transfected with or without E6 plasmid, and levels of OGT were measured by immunoblotting with an anti-OGT antibody.