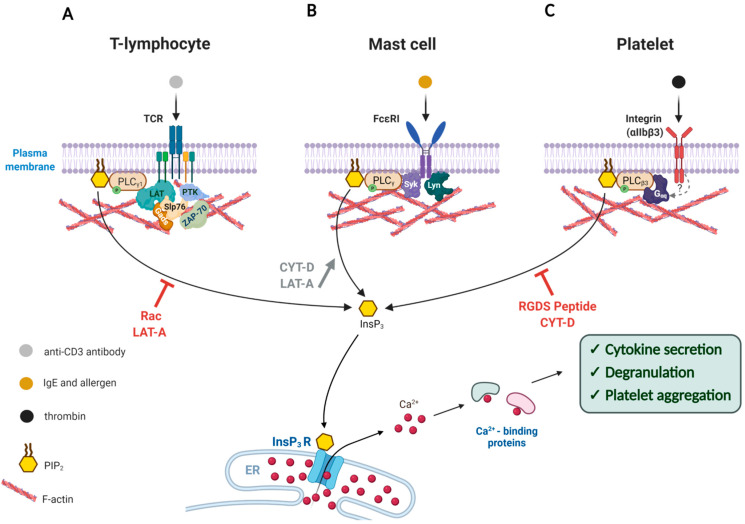
Figure 1.
Modulation of the Ca2+ signal transduction by the actin cytoskeleton in the cells of immune response. Artificially altered actin dynamics during cell activation either enhances or represses Ca2+ signaling through the PLC/InsP3 pathway. (A) Exposure of T-lymphocytes to anti-CD3 antibody triggers T-Cell Receptor activation that leads to a cascade of downstream phosphorylation reactions that activates enzymes like PLC-γ1, ZAP-70 (Zeta-chain-associated protein kinase 70), PTKs as well as adaptor proteins such as LAT, Slp76, and Gads. Interference with the actin dynamics by use of Rac overexpression or Latrunculin-A (LAT-A) reduces phosphorylation of these enzymes, and thereby inhibits the PLC-γ1 activity and cytokine secretion. (B) Upon binding to antigen-IgE, the FcεRI receptor on the surface of a mast cell is phosphorylated and recruits more Lyn kinase to activate Syk kinase, which in turn stimulates PLC-γ to produce InsP3 and thereby release intracellular Ca2+ and trigger degranulation. If the concomitantly occurring actin polymerization is prevented by actin drugs LAT-A or Cytochalasin-D (CYT-D), the activation of PLCγ is even more enhanced. (C) Binding of thrombin to the integrin receptor (αIIbβ3) on the plasma membrane leads to enhanced polymerization of actin, which is accompanied by the translocation of PLCβ3 and other proteins. The resulting Ca2+ signal induces platelet aggregation. When the responsive actin polymerization is inhibited by CYT-D or fibrinogen antagonist tetra-peptide (RGDS), the extent of PLCγ activation and platelet aggregation is severely compromised. Note: The T-shaped red bars near the curved arrows denote inhibition.