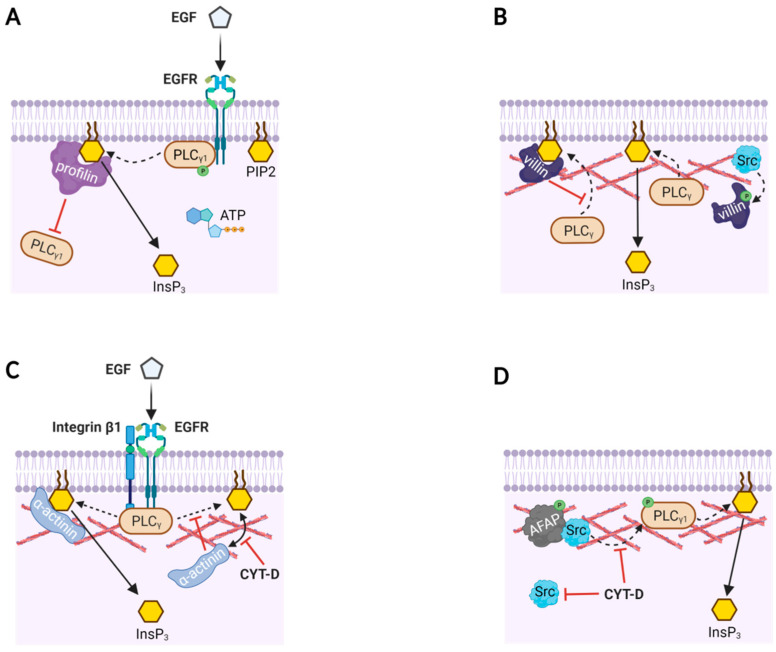
Figure 2.
Actin-binding proteins modulate PLC activities with or without actin filaments. (A) A model based on in vitro reaction mixture containing phospholipid bilayer with PIP2, purified EGFR, PLC-γ1, ATP, and profilin. Profilin binds to PIP2, and thereby prevents PLC-γ1 from accessing its substrate PIP2. When PLC-γ1 is phosphorylated by the activation of EGFR, the inhibition by profilin is overcome and PIP2 is hydrolyzed to produce InsP3 and DAG (not shown). (B) Likewise, villin masks PIP2 from PLC-γ, but phosphorylated villin (by Src) loses its binding affinity to PIP2. Then, PLC has access to PIP2 to produce InsP3. (C) The focal adhesion complex in human mammary epithelial (HME) cells comprises PLC-γ, integrin β1, α-actinin, F-actin, and EGFR. Binding of EGF to EGFR induces EGF receptor dimerization, phosphorylation of PLC-γ, and increased α-actinin loading on PIP2. Here, α-actinin’s binding to PIP2 facilitates its hydrolysis by PLC-γ, and consequently the production of InsP3 doubles. Disruption of the actin cytoskeleton with Cytochalasin-D (CYT-D) dissociates α-actinin from PIP2, and the EGFR-associated PLC-γ is unable to cleave the PIP2 not bound to α-actinin. (D) Actin filament-associated protein (AFAP) in rat fetal lung cells presents Src to PLC-γ1 via actin filaments so as to activate the enzyme. As a result, InsP3 production is increased, but the effect is inhibited by CYT-D.