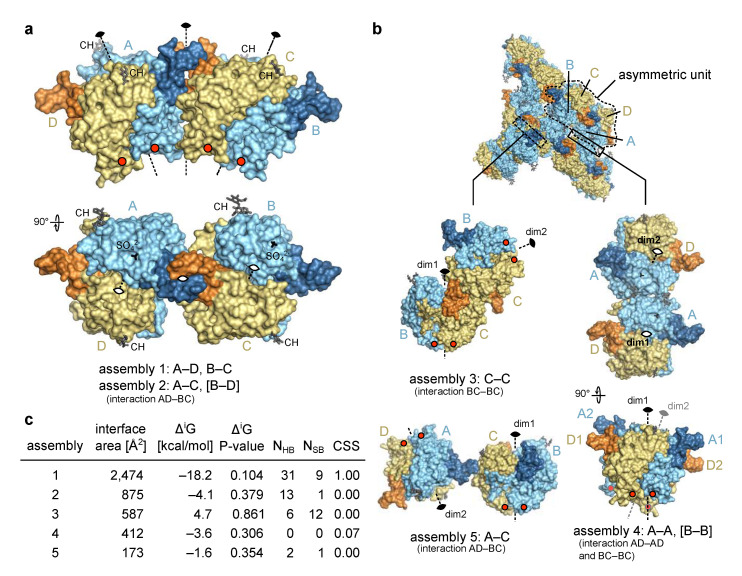
Figure 2.
Molecular assemblies within the asymmetric unit and beyond. (a) In asymmetric unit, four polypeptide chains (chains A to D) corresponding to four copies of Trop2 ectodomain were found and are shown here as molecular surface. Chains A and B are shown in pale cyan and their N-terminal domains in dark blue, while chains C and D are shown in light yellow and their N-terminal domains in pale orange. Improper rotational (non-crystallographic) symmetry axes are shown as dashed lines and denoted with the standard 2-fold symmetry symbol. Sulfate ions (SO42−, only in chains A and B) and N-linked carbohydrates (CH) are shown as black and dark grey sticks, respectively. Red dots indicate positions of C-termini. Interaction in square brackets is not shown, however it is implied by symmetry. (b) Alternative assemblies as devised from crystal contacts and with interface area larger than 150 Å2. Color-coding is the same as in (a). Dim1 and dim2 denote two-fold axis of each dimer. Interaction in square brackets is not shown, however it is implied by symmetry. (c) Table of assemblies and associated interface area, ΔiG and associated P-value, number of hydrogen bonds (NHB) and salt bridges (NSB) at the interface, and CSS as reported by PISA [38]. In case of two equivalent interfaces average values are reported, for example as in assembly 4.