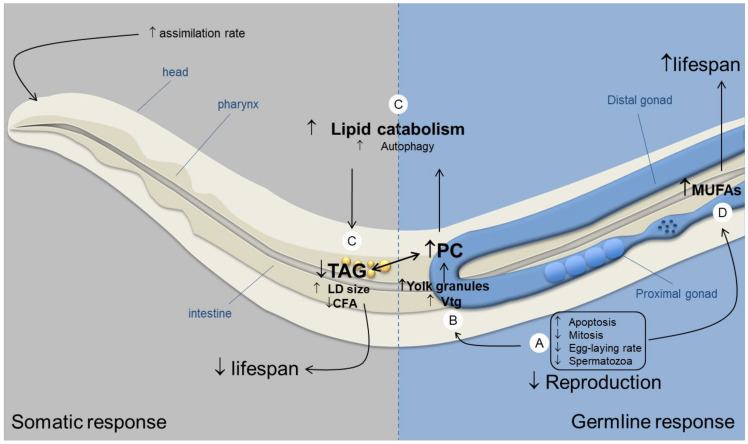
Figure 7.
Proposed model for germline and soma regulation of lipid homeostasis after chronic exposure to IR during whole development in C. elegans N2 hermaphrodite (↑ corresponds to an increase, ↓ corresponds to a decrease). IR induce germline defects (A) with the reduced egg-laying rate, brood size and sperm number (present study), the increase of radio-induced apoptosis and cell-cycle arrest [11] and reduced mitotic cell number (results not shown). This germline deficiency and/or the stress-induced intestine autophagy [22] could lead to (B) an accumulation of yolk granules as shown by the increased PC content (present study) and overexpression of vitellogenins (vtg) [3]. In turn, this accumulation of fat content could lead to lipid intoxication, known to reduce lifespan [74]. In response to this accumulation, the soma acts to regulate lipid levels by (C) increasing autophagy and lipid catabolism (probably through the IIS pathway) as shown by the decreased TAG and CFA content (present study), and the upregulation of genes involved in autophagy after IR exposure [19]. Autophagy is known to reduce lipid intoxication, thus enhancing lifespan [75]. However, under stress conditions, previous studies have shown that lipid catabolism was associated with decreased lifespan in germline less mutants [27,35]. However, in N2 strains, longevity is not affected by IR under our conditions [8,27]. Therefore, either lipid catabolism is also detrimental to lifespan, or the germline provides an opposite signal that induces an increase of longevity, compensating the positive response of the soma, as observed through the increase of MUFA content (D) (present study), known to be regulated by the germline [55,57] and to enhance longevity [37,38].