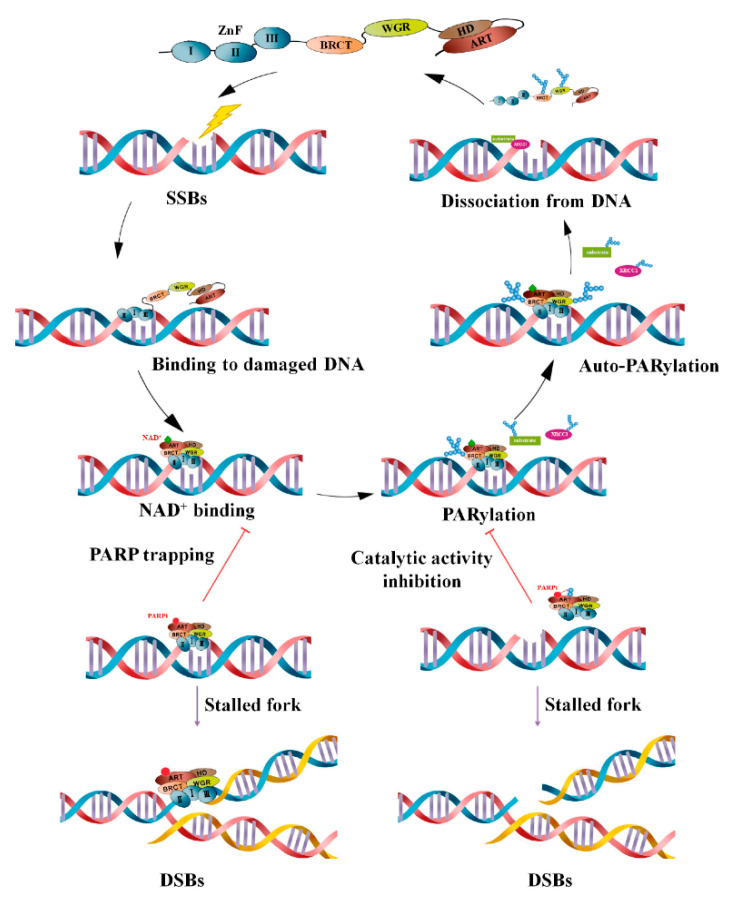
Figure 1.
Mechanisms of PARP-mediated PARylation following the occurrence of a single-strand break (SSB). PARP enzymes (specifically PARP-1) are promptly recruited to the damaged site with the help of a zinc finger domain. By using NAD+ as substrate, PARylation enhances the recruitment of DNA damage proteins, including X-ray repair cross complementing group 1 (XRCC1), DNA ligase III, etc. Auto-PARylation of PARP diminishes its affinity for DNA, and PARP enzymes dissociate from DNA. As competitive inhibitors, PAPR inhibitors could bind to the pocket instead of NAD+, a situation generally referred to as PARP trapping. PARPs trapping at the replication fork leads to aggregation of unrepaired SSBs and double-strand breaks (DSBs) and ultimately induces cell death in cancer cells. Reprinted from ref. [61].