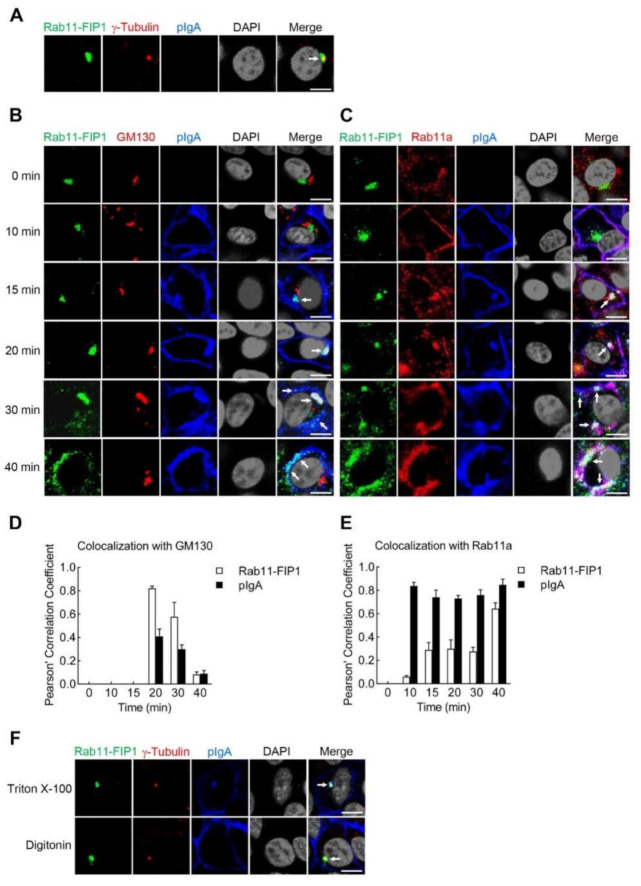
Figure 5.
Rab11−FIP1 colocalizes with pIgA during pIgA transcytosis from the vicinity of centrosome to apical plasma membrane. (A) Colocalization of Rab11−FIP1 with the centrosome was detected. Vero−pIgR cells (1 × 105) were grown on Transwell for 3 days. Cells were fixed with 4% paraformaldehyde and stained with the indicated antibodies before observation by confocal microscopy. Scale bar: 10 µm. (B,C) Colocalization of Rab11−FIP1, pIgA with the Golgi apparatus (GM130) or recycling endosomes (Rab11a) was detected. Vero−pIgR cells (1 × 105) were grown on Transwell for 3 days. An amount of 20 µg pIgA was added or not added to the basal chamber for 10 min at 37 °C and cells were then washed three times. Subsequently, cells were cultivated at 37 °C and harvested at the indicated time points. Finally, cells were fixed with 4% paraformaldehyde and stained with the indicated antibodies before observation by confocal microscopy. Scale bar: 10 µm. (D,E) Quantitative analysis of colocalization of Rab11−FIP1, pIgA with GM130 or Rab11a. Statistical analysis was based on colocalization images (covering dozens of cells) using the ImageJ software. (F) Analysis of localization of Rab11−FIP1 on the endosomes containing pIgA. Vero−pIgR cells (1 × 105) were grown on Transwell for 3 days. An amount of 20 µg pIgA was added to the basal chamber for 15 min at 37 °C and cells were then washed three times. Cells were fixed with 4% paraformaldehyde and then were permeabilized by 0.1% Triton X−100 or 20 µg/mL digitonin for 10 min at 4 °C. Finally, cells were stained with the indicated antibodies before observation by confocal microscopy. Scale bar: 10 µm. Data of (A–F) are representative of three independent experiments.