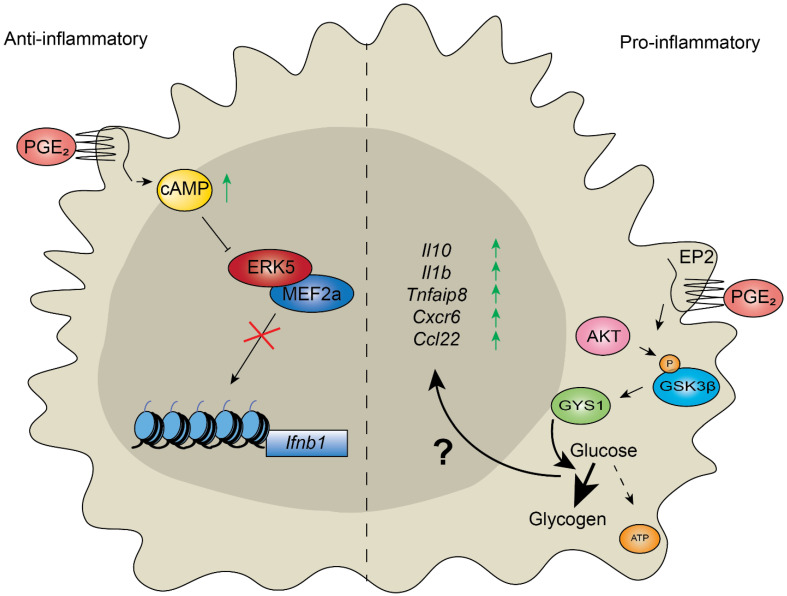
Figure 3.
Prostaglandin E2 has context-dependent effects on inflammatory response in macrophages. (Left) Modulation of inflammation in human- and mouse-derived macrophages by Prostaglandin E2 (PGE2) stimulates increased cyclic adenosine monophosphate (cAMP) accumulation [55]. The transcription factor Myocyte Enhancer Factor 2A (MEF2a) activates the transcription of many lipopolysaccharide (LPS)-inducible genes in macrophages, including pro-inflammatory genes. The treatment of LPS-stimulated macrophages with PGE2 triggers the accumulation of cAMP, which prevents Extracellular signal-regulated kinase 5 (ERK5)-activated MEF2a recruitment to pro-inflammatory genes and therefore suppresses an inflammatory response. (Right) PGE2 promotes inflammation in aged microglia [60]. Increased levels of PGE2 in aged cells are sensed by the Prostaglandin E₂ receptor 2 (EP2), which leads to AKT-mediated phosphorylation of glycogen synthase kinase 3 β (GSK3β) at serine 9, leading to its inactivation. GSK3β is a negative regulator of glycogen synthase (GYS1), which promotes glycogen accumulation from glucose, reducing the levels of glucose-derived metabolites entering the TCA cycle (glucose flux, dashed arrow). Reduced glucose flux in aged macrophages promotes the transcription of pro-inflammatory genes by an unknown mechanism (“?”). Inhibition of the EP2 receptor, or GYS1, restores macrophage phenotypes to a more youthful state. ↑ indicates increased cAMP/ gene expression, × indicates inhibition.