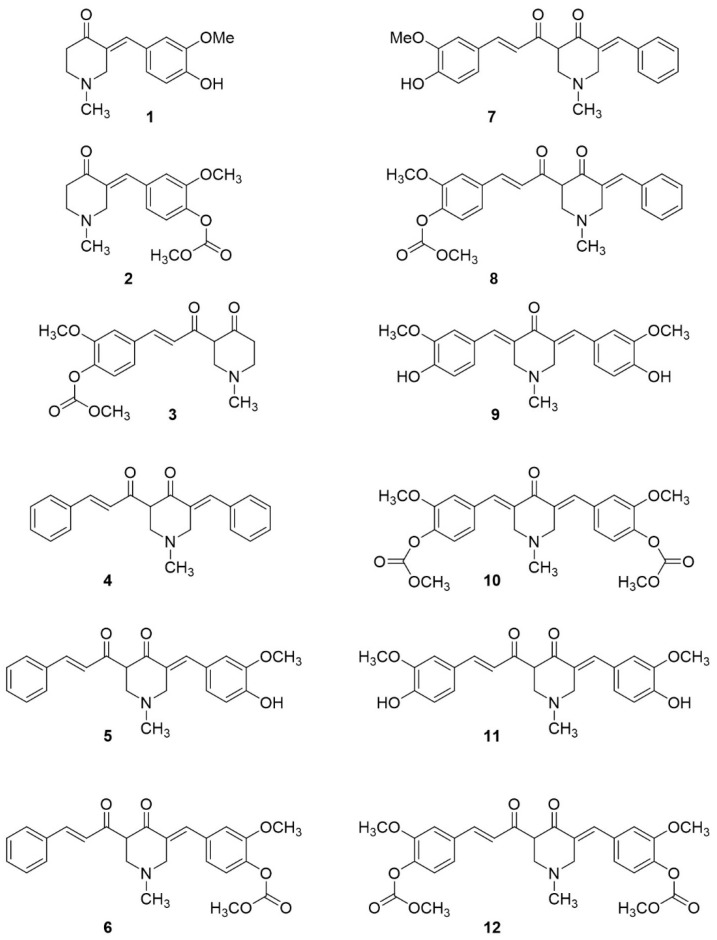
Figure 2.
Structures of the obtained curcumin analogues. The first step of the synthesis was the aldol reaction of 4-piperidone and selected aldehydes (benzaldehyde or 3-methoxy-4-methoxycarbonyloxy-benzaldehyde) promoted by lithium diisopropylamide (LDA) in anhydrous tetrahydrofuran (THF). The obtained aldols were dehydrated (elimination of water) in acidic conditions to obtain monosubstituated benzylidene piperidones. We chose a complicated procedure using LDA in anhydrous conditions to avoid obtaining only the thermodynamically stable product of attachment of two aldehyde molecules, the formation of which was observed under both acidic and basic conditions [16,26]. In the synthesis of compound 2, the hydroxyl group of aldol was acetylated (catalyzed by DMAP: 4-(dimethylamino)pyridine) before acid-catalyzed elimination. This transformation allows one to perform the elimination reaction under milder conditions (without heating) and increase the yield of obtained product [25]. Unfortunately, despite those preventive measures, in addition to the desired product 2 (29% yield), 2,4-disubstituated piperidone 10 (14% yield) was obtained (Scheme 1).