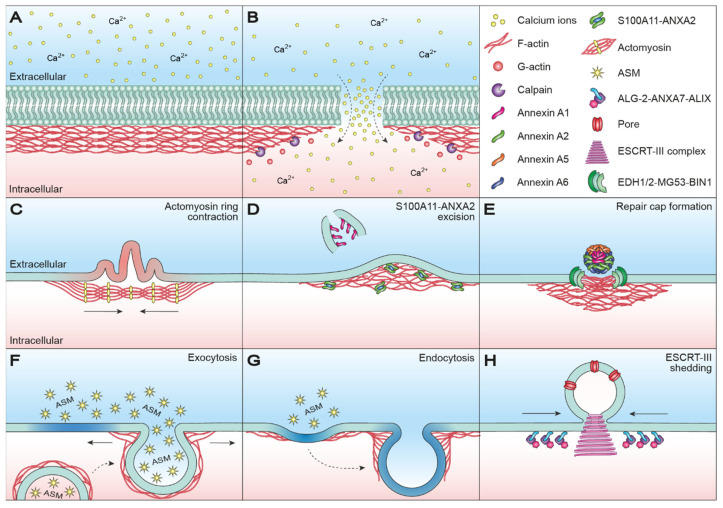
Figure 1.
Actin-associated plasma membrane repair mechanisms in single-cell wound repair. (A) The PM and underlying cortical actin cytoskeletal are intact and maintaining cellular homeostasis. (B) Disruption of the PM causes a rapid influx of Ca2+, which causes depolymerization of actin filaments to G-actin monomers primarily through Ca2+-dependent activation of intracellular calpain proteases. Moreover, the increase in intracellular Ca2+ activates and recruits repair machinery for membrane resealing. (C) Subsequent to membrane patching, PM restoration in Xenopus oocytes occurs by contraction of an actomyosin ring anchored to the uninjured membrane at regular intervals. Ring contraction pushes injured membrane (red) outward, while dragging uninjured membrane (green) inward. (D) S100A11-ANXA2-dependent actin polymerization promotes wound closure by pulling the membrane edges towards each other for resealing and excision of injured membrane marked by ANXA1. (E) In muscle cells, formation of an annexin-rich repair cap, consisting of ANXA1, ANXA2, ANXA5 and ANXA6, and an underlying clearance zone of F-actin are required for efficient sarcolemma repair. An adjacent shoulder of repair proteins, EDH1/2, MG53 and BIN1, further aids the repair process. (F) Injury-induced lysosome-exocytosis reduces in-plane tension and generates ceramide microdomains (blue) through secretion of ASM. Mesoscopic modulation of the actin cytoskeleton is a prerequisite for vesicular exocytosis, since cortical actin comprises a mechanical barrier preventing vesicle fusion. At the same time, vesicle fusion cannot occur without some degree of actomyosin-mediated assistance. (G) ASM-generated ceramide microdomains (blue) trigger membrane invaginations, thereby priming the membrane for endocytosis-mediated restructuring. Because several steps of the endocytic process require delicate actin modulation, the internalization of injured membrane regions is highly dependent on actin dynamics. (H) ESCRT-III-mediated shedding of pore-injured membrane regions through spiral contraction. Ca2+-dependent formation of ALG-2-ANXA7-ALIX complexes at the injury site promotes the sequential recruitment of ESCRT-III components, resulting in complex assembly and microparticle shedding. ANXA: annexin; ALG-2: apoptosis-linked gene-2; ALIX: ALG-2-interacting protein X; ASM: acid sphingomyelinase; BIN1: Myc box-dependent-interacting protein 1; Ca2+: calcium ions; EDH1/2: EH-domain-containing protein 1/2; ESCRT-III: endosomal sorting complex required for transport III; G-actin: globular actin; MG53: Mitsugumin 53; PM: plasma membrane; S100A11: S100 calcium binding protein A11; Vps4B: vacuolar protein sorting-associated protein 4B.