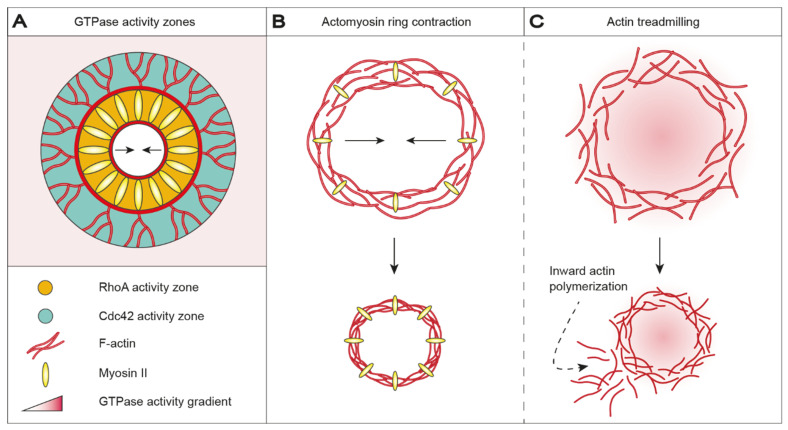
Figure 2.
Wound healing in Xenopus oocytes is accomplished by contraction of an actomyosin ring or actin treadmilling. (A) Actomyosin ring positioning, assembly and contraction are orchestrated by demarcated GTPase activity zones. Injury-triggered Ca2+ influx recruits and activates RhoA (orange) and Cdc42 (green), which accumulates in mutually exclusive, concentric activity zones around the wound. RhoA and Cdc42 co-localize with myosin II and actin, respectively. RhoA stimulates polymerization of stable, unbranched actin filaments in the inner zone, whereas Cdc42 stimulates polymerization of dynamic, branched actin filaments in the outer zone. (B) In detail, RhoA controls actomyosin contractibility by influencing the activity of MLCK and ROCK, which in turn regulates myosin II light chain phosphorylation levels. Accordingly, wound closure is promoted by actomyosin-based ring contraction. (C) Alternatively, contraction-independent wound closure can be mediated through actin treadmilling. Here, a directional GTPase activity gradient from the leading edge to the trailing edge of the wound results in inward actin polymerization, which constricts and eventually seals the wound; however, actin treadmilling results in less organized and discontinuous wound closure. Ca2+: calcium ions; Cdc42: cell division control protein 42 homolog; MLCK: myosin light-chain kinase; RhoA: Ras homolog family member A; ROCK: Rho-associated kinase.