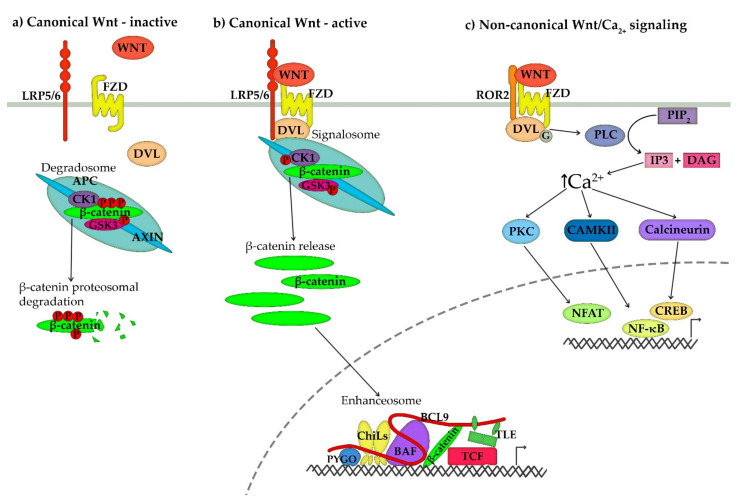
Scheme 1.
Schematic representation of (a) the inactive Wnt signalling pathway, (b) activated canonical Wnt signalling pathway and (c) non-canonical Wnt/Ca2+ signalling pathway. (a) In the absence of a Wnt ligand, the canonical Wnt signalling pathway is inactive, and β-catenin is degraded by a multiprotein complex termed axin degradosome. Degradosome is composed of scaffold proteins adenomatous polyposis coli (APC) and axin and kinases glycogen synthase kinase 3 (GSK3) and casein kinase 1 (CK1). The two kinases phosphorylate β-catenin and release it into the cytosol, where it is ubiquitinated and degraded by a proteasome complex. (b) Canonical Wnt signal transduction is initiated by the binding of Wnt ligand (WNT) to membrane frizzled (Fzd) receptor and low-density lipoprotein receptor-related protein 5/6 (LRP5/6) co-receptor. Fzd and LRP5/6 undergo dimerization and conformational changes, which recruit dishevelled (DVL) and degradosome to the membrane and trigger the assembly of a multiprotein complex termed signalosome. Inhibition of kinases releases β-catenin from the complex and enables its accumulation in the cytosol and translocation to the nucleus. In the nucleus, β-catenin incorporates into a multiprotein complex termed enhanceosome, composed of Pygopus (PYGO), ChiLs complex, adaptor protein B-cell lymphoma 9 (BCL9) and BAF (Brg/Brahma-associated factors) chromatin-remodelling complex. The main proteins of enhanceosome are transcriptional co-repressors TLE (transducin-like enhancer of split) and T cell factor (TCF) family of DNA-bound transcription factors, which mediate transcription of β-catenin dependent genes through changes in histone acetylation and chromatin condensation. (c) Non-canonical Wnt signalling is activated by binding a non-canonical Wnt ligand to Fzd receptor and Receptor tyrosine kinase-like orphan receptor 2 (ROR2). Receptor dimerization recruits DVL to the membrane and activates heterotrimeric G-proteins, which in turn activate phospholipase C (PLC). The PLC cleaves the membrane-bound phosphatidylinositol-4,5-bisphosphate (PIP2) into inositol-1,4,5-trisphosphate (IP3) and 1,2 diacylglycerol (DAG). IP3 induces the release of Ca2+ from intracellular calcium stores and activation of calcium-sensitive enzymes, such as protein kinase C (PKC), calmodulin-dependent protein kinase II (CaMKII) and calcineurin. These activated proteins, in turn, activate several transcription factors, such as nuclear factor κB (NF-κB), cAMP-responsive element-binding protein (CREB) and nuclear factor associated with T cells (NFAT).