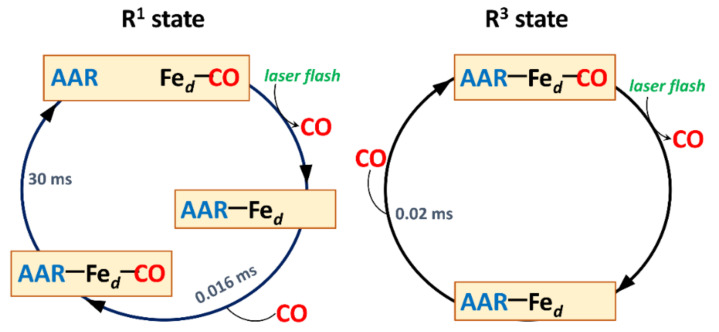
Figure 6.
Proposed scheme for a cycle of photolysis and recombination of CO to bd-type oxidase in one-electron-reduced (R1) and fully reduced (R3) states. In the R1 state, photodissociation of CO from the ferrous heme d iron (Fed) is accompanied by binding of the axial ligand, an amino acid residue (AAR), at the opposite side of the heme. CO rebinds to Fed with τ of 0.016 ms producing a transient hexacoordinate state (AAR–Fed–CO). Then AAR is dissociated from Fed with τ of 30 ms. In the R3 state, AAR is a permanent indissociable ligand to Fed. Photodissociation of CO from Fed is followed by its rebinding to the heme with τ of 0.02 ms. AAR is either H19 [34] or E99 [35]. The scheme is based on time-resolved spectroscopic studies [47,48,49]. The electron transfer in the opposite direction (back-flow) observed in the R1 state occurs to a much lesser extent than in heme-copper oxidases and therefore is not shown in the scheme.