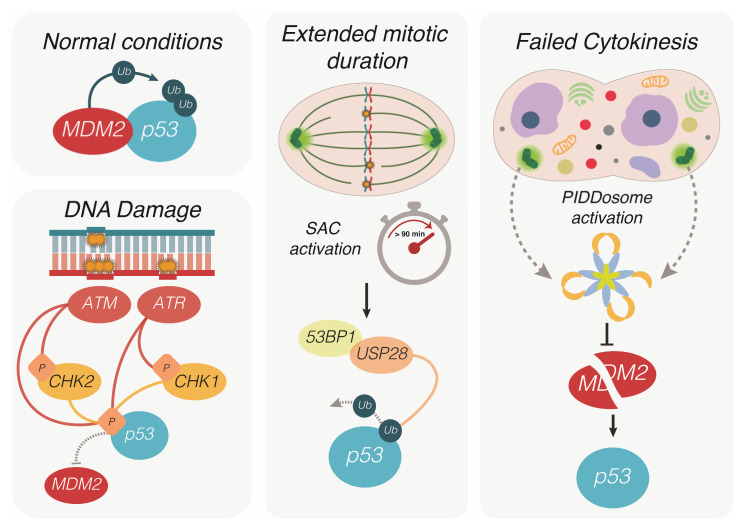
Figure 1.
Different routes leading to p53 activation. In physiological conditions, p53 is bound and ubiquitinated by its negative regulator MDM2, which prevents nuclear translocation and promotes its proteasomal degradation. Upon single strand or double strand DNA damage, the kinases ATR and ATM activate the checkpoint kinases Chk1 and Chk2, which contribute to p53 phosphorylation on specific amino acidic residues. Phosphorylated p53 can no longer be bound and degraded by MDM2, resulting in protein stabilization, nuclear translocation and the activation of its transcriptional program. During mitosis, prolonged prometaphase due to the activation of the spindle assembly checkpoint (SAC) is sensed by 53BP1 and USP28. The latter protein promotes de-ubiquitination of p53, arresting the cell cycle in the next interphase. Defective cytokinesis prevents daughter cells to separate completely at the end of mitosis, resulting in a single polyploid cell containing extra centrosomes. The multiprotein complex PIDDosome senses the presence of extra centrosomes and functions as an activating platform for caspase-2. Being a target of this protease, MDM2 is cleaved and thereby inactivated, resulting in the accumulation of p53 and cell cycle arrest of the polyploid cell.