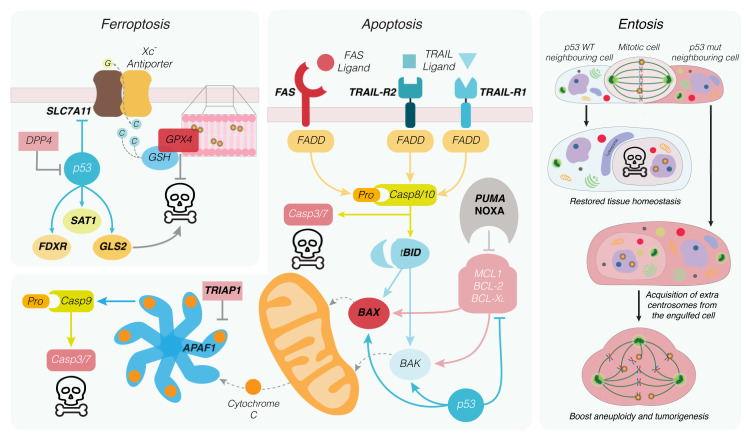
Figure 3.
Critical p53-regulated cell death modalities. Upon alterations of the cellular redox state, iron-dependent peroxidation of membrane phospholipids can occur, promoting ferroptosis. Peroxidation can be reversed by the selenocysteine-containing protein glutathione peroxidase 4 (GPX4) in a glutathione (GSH)-dependent manner. By inhibiting the transcription of the system Xc- complex subunit SLC7A11, p53 reduces the reservoir of cysteine (C) necessary for GSH synthesis, thus preventing reversion of lipid peroxidation. Moreover, p53 controls the transcription of FDXR, SAT1 and GLS2, favouring cell death via ferroptosis. Extrinsic apoptosis is initiated by e.g. FAS or TRAIL ligand binding to their cognate receptors promoting the multimerization of receptor trimers. These form the cell death inducing signalling complex (DISC) that recruits the adapter protein FADD, which promotes activation of the initiator caspase-8, which activates in turn executioner caspases-3 and -7, alongside the BCL2 family protein BID. Intrinsic apoptosis depends solely on the balance between the inhibitory (e.g. MCL1, BCL2, BCLX) and the apoptosis-promoting members of the BCL2 family. Regardless of the apoptotic route, BAX and BAK homo-oligomerization in the mitochondrial outer membrane results in release of cytochrome C, which complexes to APAF1 forming the apoptosome. This multiprotein complex allows dimerization of the initiator caspase-9, which in turn activates the cytosolic executioner caspases-3 and -7, leading to cell death. Cells experiencing defective mitosis are prone to form cell in cell (CiC) structures with neighbouring cells in a process called entosis. In a p53 wildtype context, the internalized cell is killed by lysosomal degradation, preventing the outgrowth of defective cells. On the contrary, entosis fosters tumorigenesis in p53 mutated cells by interfering with the mitotic process of the internalizing cell and promoting aneuploidy. Direct transcriptional targets of p53 are labelled in bold.