Abstract
Angiogenesis plays a key role in the wound healing process, involving the migration, growth, and differentiation of endothelial cells. Angiogenesis is controlled by a strict balance of different factors, and among these, the angiogenin protein plays a relevant role. Angiogenin is a secreted protein member of the ribonuclease superfamily that is taken up by cells and translocated to the nucleus when the process of blood vessel formation has to be promoted. However, the chemical signaling that activates the protein, normally present in the plasma, and the transport pathways through which the protein enters the cell are still largely unclear. Copper is also an angiogenic factor that regulates angiogenin expression and participates in the activation of common signaling pathways. The interaction between angiogenin and copper could be a relevant mechanism in regulating the formation of new blood vessel pathways and paving the way to the development of new drugs for chronic non-healing wounds.
Keywords: angiogenesis, trophic factor, ribonuclease, cell proliferation, protein, mimicking peptides, copper complexes
1. Introduction
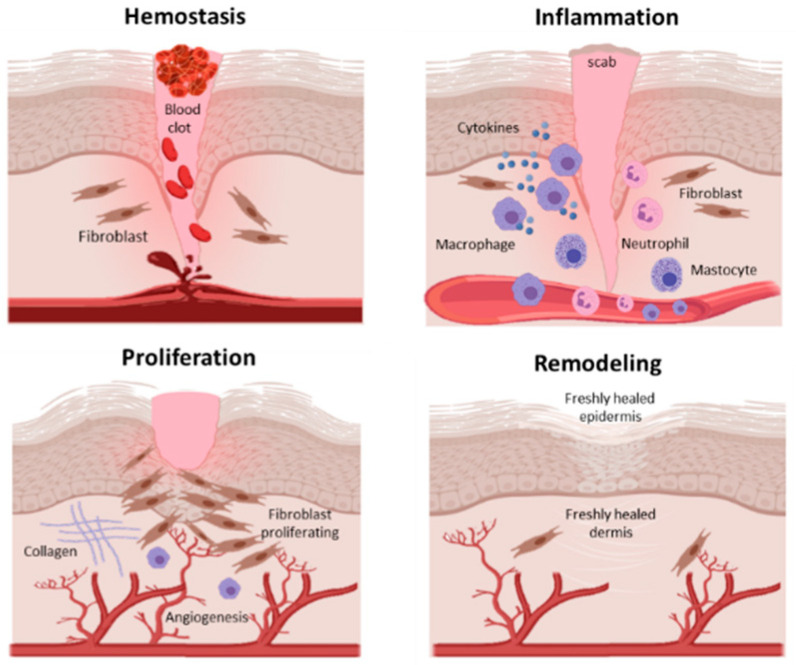
Wound healing is a complex, dynamic and finely balanced series of events starting immediately after an injury. This process includes a strictly coordinated interaction of blood cells, proteins, growth/trophic factors, proteases and extracellular matrix components [1], which takes place in four overlapping phases: (1) hemostasis; (2) inflammation; (3) proliferation; (4) remodeling [2] (Figure 1).
Figure 1.
Phases of the wound healing process: hemostasis; inflammation; proliferation; remodeling.
Hemostasis involves the control of blood loss in the damaged region by the activation of the clotting cascade and formation of a large temporary fibrin mesh that fills the wound, preventing microorganism invasion and providing a temporary matrix that serves as a scaffold structure for further healing processes [3].
After hemostasis is achieved, there is a cellular inflammatory response that plays a protective role against invading agents and supports the removal of cell debris. During the inflammatory stage, indeed, immune cells, namely mastocytes, which release cytokines as well as lysosomal enzymes and reactive oxygen species (ROS), migrate to the injury site, thus causing the appearance of oedema and erythema [4]. This process involves also different players, such as Langerhans cells, one of the primary cell types of the immunological barrier, which play an important role during the inflammatory phase of acute wound healing [5], and gamma-delta cells involved in inflammation and re-epithelialization and required for efficient skin wound healing [6,7]
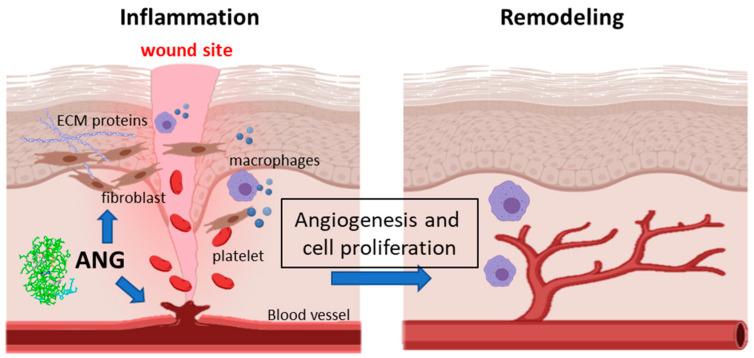
Cell proliferation requires an adequate blood supply in order to receive sufficient oxygen and nutrients [8]; therefore, the following stage is marked by epithelialization, angiogenesis, granulation tissue formation and collagen deposition [1]. This process involves several cell types, including fibroblasts, macrophages and endothelial cells, which exert interdependent activity during wound management. In particular, macrophages provide cytokines that are essential to promote both fibroplasia and angiogenesis; fibroblasts construct the extracellular matrix (ECM), which is indispensable to support the growing tissue; endothelial cells digest and penetrate the vascular basement membrane, invade the ECM and form tube-like structures, which continue to extend branches, thus creating new blood vessel networks [9]. The angiogenic response is stimulated by the release of several soluble molecules capable of regulating cell interactions and stimulating wound angiogenesis. Among them, fibroblast growth factor (FGF) [10], vascular endothelial growth factor (VEGF) [11], platelet-derived growth factor (PDGF), thrombospondin and angiogenin (ANG) play a pivotal role [12,13].
The last step of the proliferation phase consists of fibroblasts’ migration to the wound site and proliferation within the wound for granulation tissue formation. Remodeling is the last phase of wound healing, and at this stage, the maturation of the wound begins. Extracellular matrix components are partially subject to modifications; in particular, collagen type III is replaced by stronger collagen type I, to form a more organized extracellular structure. Fibroblasts and keratinocytes are two cell lines strictly involved in this process, and their interaction, via a paracrine loop, is essential to the outcome of successful dermal remodeling and the transition from granulation tissue to scar formation [14].
Taking into account the complexity and interconnection of the molecular mechanisms involved and the time required for the wound healing process to be fully completed, it is clear that any serious imbalance in the wound healing phases can lead to dysfunction, such as excessive wound healing or chronic wound formation [15,16].
The pathogenesis of excessive wound healing is not fully understood. It is an abnormal form characterized by continuous, localized inflammation in the wound region, which results in excessive collagen synthesis and an overstated accumulation of extracellular matrix components in these wounds. Examples of excessive wound healing are keloid and hypertrophic scars [17].
Keloid scars are characterized by their extensive growth beyond the borders of the original wound, whereas hypertrophic scars are defined as visible and elevated scars that do not spread into surrounding tissues. The precise reason that wound healing sometimes leads to keloid or hypertrophic scar formation is not yet fully elucidated. Both scar types may be uncomfortable but are generally harmless to individual health [18,19,20]. In contrast, pathologies linked to an unhealed wound may be more dangerous.
A wound that has failed to heal in four weeks is defined as a chronic wound. Generally, it is associated with underlying pathologies of a more diverse nature, such as cancer, malnutrition or vascular pathologies. Today, diabetes represents a pathology for which chronic wound healing is a major concern. Diabetic foot ulcers are among the most common complications of patients who have diabetes mellitus and precede the vast majority of amputations in this patient population [21].
Therefore, in diabetes and vascular pathologies, wound care has become increasingly relevant given the rise in chronic wounds and the morbidity associated with them.
A common side effect of injured skin is the possible infection, and many wound dressings have been developed in order to further protect the healing wound from infection and to promote the wound healing process itself.
In this review, we will focus on the role of the angiogenin protein, a potent endogenous angiogenic factor that exhibits antimicrobial properties and an ability to bind copper ions [22,23,24]. Copper is also a known angiogenic factor that regulates different steps of the wound healing process, including the expression of VEGF and ANG [25,26]. Therefore, the potential interconnection between ANG and copper in wound healing processes will be discussed.
2. Angiogenin Structure
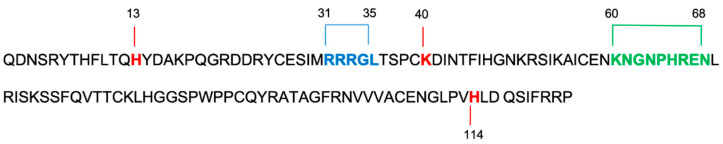
ANG is a 14,200 Da basic single-chain protein, a member of the RNase family, and is physiologically present in the blood plasma at concentrations of 200–400 ng/mL [27]. It was first discovered and characterized by Vallee and colleagues in 1985 [28]. Its primary structure includes 123 amino acids, with 33% sequence identity and 65% sequence homology with respect to pancreatic ribonuclease A (RNase A). Similarly to RNase A, ANG shows a “kidney-shaped” structure consisting of three α-helices and seven β-sheets stabilized by three disulphide bridges involving the residues Cys-26-Cys-8, Cys-39-Cys-82 and Cys-57-Cys-107, respectively [29]. ANG, as well as RNase A, contains both a catalytic center, formed by the triad His-13, Lys 40 and His-114 (Figure 1), and a purine/pyrimidine binding site [22]. Despite these analogies, the enzymatic activity of ANG is 105–106-fold lower compared to the ribonucleolytic activity of RNase A. The rationale behind the low enzymatic activity of ANG is the obstruction of its pyrimidine base binding site by the glutamine residue, Gln-117, which forms two hydrogen bonds with the threonine residue, Thr-44 [30]. The position of Gln-117 is also determined by intramolecular hydrophobic interactions, which involve the amino acid residues isoleucine, Ile-1 and Ile-19, and phenylalanine, Phe-120 [31]. Accordingly, mutation of the Gln-117 residue leads to an increase in the enzymatic activity of ANG [30]. Furthermore, ANG lacks the fourth disulphide bridge, with respect to RNase A, resulting in the formation of a loop region, including the amino acid sequence 60–68, which is involved in the endothelial cell surface receptor interaction [32]. The ANG structure comprises, also, a nuclear localization sequence encompassing the amino acid sequence 30–35 (Figure 2) [33].
Figure 2.
Amino acid sequence of angiogenin using one-letter symbols. The first residue Q, glutamine, is cyclized as pyroglutamate. Red indicates amino acids of catalytic sites (H, histidine; K, lysine); blue indicates nuclear translocation sequence (R, arginine; G, glycine; L, leucine); green indicates cellular binding site (K, lysine, N, asparagine; G, glycine, P, proline, H, histidine; R, arginine; E, glutamic acid).
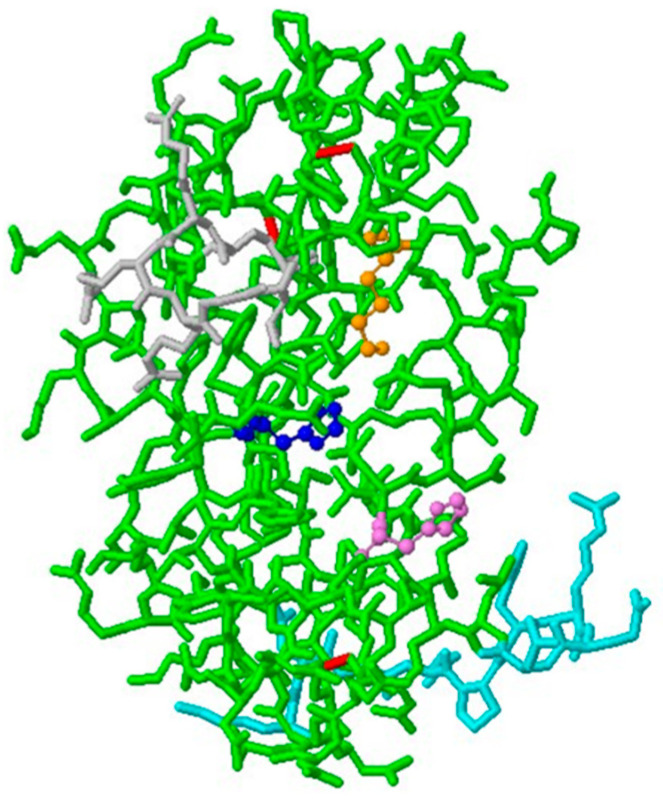
The catalytic site, the receptor binding site and the nuclear translocation sequence constitute the three characteristic functional sites of ANG, which explain its unique RNase activity and allow for its distinctive biological functions, as a key factor in blood vessel formation [34] (Figure 3).
Figure 3.
Three-dimensional biological structure of human ANG, consisting of: three disulphide bridges (in red); a catalytic site formed by the triad His-13 (in blue), Lys-40 (in orange) and His-114 (in violet); the receptor binding site, sequence 60–68 (in cyan); the nuclear translocation sequence, 30–35 (in grey). (PDB ID 1ANG, 10.2210/pdb1ANG/pdb).
Interestingly, the integrity of all three functional sites is essential for the maintenance of the biological activity of ANG, since it has been demonstrated that mutation of the His-114 residue causes the complete loss of both the enzymatic activity of the protein and its angiogenic action [24].
2.1. Angiogenin Activity
The angiogenic action of ANG is based on protein–protein interactions through which angiogenin promotes endothelial cells’ growth, survival, migration and invasion. Based on data from the literature, ANG binds to a 170 kDa transmembrane receptor located on the surface of angiogenin-responsive endothelial cells. The interaction between ANG and its receptor induces conformational changes in the protein, moving the glutamine Gln-117 residue from its obstructive position and allowing ANG to bind to its natural substrate RNA, and triggers several signal transduction pathways, through the activation of secondary messaging cascades [35].
Recently, plexin-B2 has been identified as a functional angiogenin binding receptor on activated endothelial cells [36].
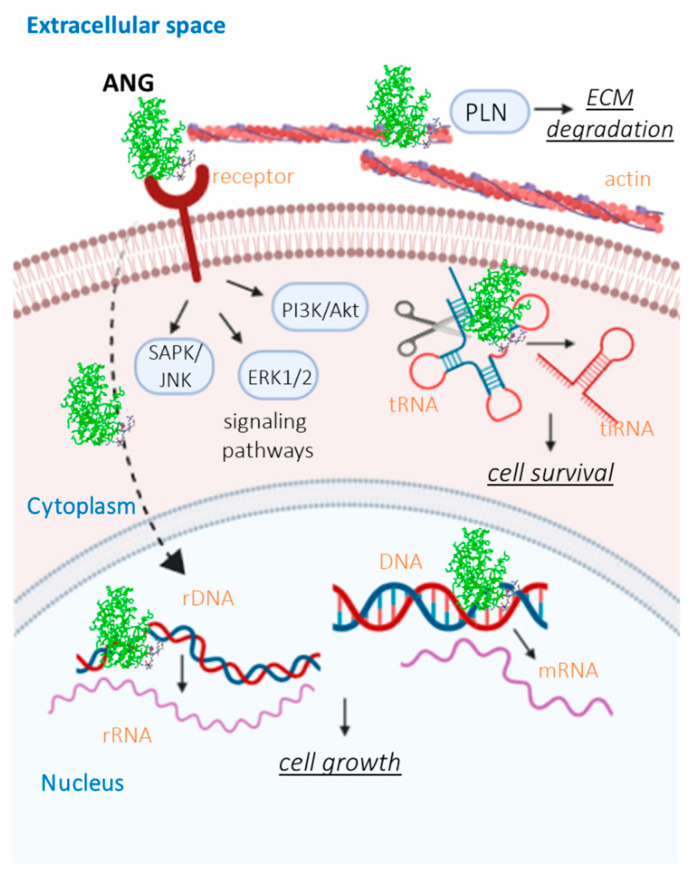
As result of protein cellular recognition, ANG stimulates signaling pathways related to extracellular signal-regulated kinase 1/2 (ERK1/2) [37], serine/threonine-protein kinases (B/Akt) [38] and stress-associated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) [39], increasing the production of intracellular ribosomal proteins and enhancing cell growth and proliferation. In addition, through the activation of the phosphatidylinositol-3 kinase/Akt pathway (PI3K/Akt), ANG promotes the synthesis and release of nitric oxide, a vasodilator factor, involved in the vascular physiology [40]. Along with the activation of extracellular transduction pathways, ANG is able to pass through the cell membrane, via “receptor-mediated endocytosis”, and accumulates into the nucleus or cytoplasm of endothelial cells, under growth or stress conditions, respectively. Data reveal that inside the nucleus, ANG promotes ribosomal DNA (rDNA) transcription, thus increasing the 47S ribosomal (rRNA) levels by binding to the angiogenin binding element (ABE) on the rDNA promoter, where angiogenin induces methylation and histone modification [41]. Furthermore, ANG enhances the messenger RNA (mRNA) transcription of several genes, since it acts as a chromatin remodeling activator. In this regard, chromatin immunoprecipitation chip assays identified 699 genes that could be regulated by nuclear ANG and most of these genes are significantly expressed in tumorigenesis [42]. On the other hand, the cytoplasmic ANG, following stress conditions (e.g., oxidative damage and starvation stress), cleaves transfer RNA (tRNA) molecules, leading to the production of stress-induced tRNA-derived (tiRNA) [43] molecules, which guide protein translation, thereby promoting damage repair and cell survival.
It is also known that ANG, through its cell surface receptor binding site, forms a complex with the endothelial cell surface α-actin [44]. The relevance of this protein domain is further confirmed by the mimic role of a peptide encompassing the amino acid sequence 60–68, which interacts with actin in a similar way to the whole ANG protein [45]. α-actin binding is a crucial step for the promotion of angiogenesis since this complex is able to activate the plasminogen activator/plasmin serine protease system, leading to plasmin (PLN) generation from plasminogen [44,46]. Plasmin, indeed, is an enzyme able to degrade both laminin and fibronectin in the basement membrane and the extracellular matrix, thus promoting endothelial cell migration and invasion into the perivascular tissue, which is a crucial phase of vessel growth [47]. Furthermore, upon interaction with actin, ANG induces changes in the cell cytoskeleton by inhibiting the polymerization of G-actin and changing the physical properties of F-actin, respectively [44]. These events severely alter the cells’ mechanical properties, thus inducing strong effects on the cellular structure and function, tissue morphogenesis as well as the whole angiogenic process [22] (Figure 4).
Figure 4.
Mechanism of action of ANG. Extracellular ANG, through interaction with an endothelial surface cell receptor, activates several signal transduction pathways, including ERK1/2, SAPK/JNK and PI3K/Akt, thus promoting cell growth and differentiation. Moreover, extracellular ANG undergoes receptor-mediated endocytosis and accumulates in the cytoplasm, under stress conditions or in the nucleus, under growth conditions. Nuclear ANG stimulates both rRNA and mRNA transcription, allowing for cellular growth. In addition, ANG interacts with cell surface actin, leading to ECM and basement membrane degradation, thereby promoting cell migration and invasion.
Nevertheless, the widespread expression of ANG in several human tissues and its presence in fluids, namely plasma [48], the tumor microenvironment [49] and amniotic [50] and cerebrospinal liquids [51], suggests its participation not merely in neovascularization but also in further physiological and pathological processes, including neuroprotection [52], inflammation [53], the immune response [54], micro-biocidal activity [55] and reproduction [56]. Accordingly, mutations of the gene encoding for ANG have been found in patients affected by neurodegenerative disorders, such as amyotrophic lateral sclerosis (ALS) [57] and Parkinson’s disease (PD) [58], while an increased concentration of ANG has been measured in patients with ulcerative and Crohn’s diseases (CrD) [59].
2.2. Angiogenin and Wound Healing
As reported above, ANG is directly involved in the wound healing process as its primary biological function is blood vessel homeostasis regulation, through both the stimulation of new vessel growth and the maintenance of endothelial cell self-renewal. Furthermore, ANG activates fibroblasts and the factors that they produce, thus also indirectly influencing the course of wound healing (Figure 5).
Figure 5.
Angiogenin promotes wound healing by inducing angiogenesis and cell migration and by activating fibroblast cells to produce ECM proteins (collagen, fibrin, fibronectin).
The use of angiogenin for the topical treatment of wounds and ulcerous damage in humans has been tested and patented in Russia [60]. This is claimed to reduce the time to wound recovery and tissue generation; however, at present, and to the best of our knowledge, there are no pharmaceutical forms containing ANG for wound healing applications. An effective application requires, among other aspects, a more accurate understanding of the mechanism by which the protein promotes wound repair.
Intracutaneous injections of recombinant angiogenin in Wistar rats induce a dose effect causing morphological changes in the dermis, playing a relevant role in regenerative processes [61]. The thickness of the stratum corneum is enhanced, as well as the density of collagen fibers and the proliferation rate of epidermal cells in animals administrated with ANG compared to those used as controls. In the same work, it is demonstrated that the addition of recombinant ANG stimulates the blood cells to produce and release both pro- and anti-inflammatory cytokines, suggesting that ANG may act as a protective homeostatic factor through angiogenic process activation or through the activation in the dermal blood vessels of other circulating cells, such as lymphocytes, neutrophils and endothelial cells. Therefore, ANG may exert wound healing effects by triggering different and combined biochemical pathways in the basal layer of the dermis.
Wound healing promotion by ANG is observed in different endothelial cells. The corneal endothelial cells (CECs) form the innermost monolayer of the cornea and need to be physiologically protected against injuries [62]. A scratch wound assay carried out on CECs shows that ANG promotes cell migration and wound closure by the activation of the phosphatidylinositol 3-kinase (PI3-k) signaling pathway [63]. In the same study, the healing effect is also observed in an in vivo test. Treatment with ANG eye drops significantly reduced corneal haziness in a rabbit model of transcorneal freezing injury, in which the corneal endothelial layer was destroyed by freezing [63].
The properties of ANG have prompted the development of engineered CECs able to overexpress the protein, with the aim of mimicking the corneal endothelium in vivo and enhancing graft cellularity for transplantation approaches [64].
An important aspect of ANG’s action is its involvement in the innate immune system. The protein is also a component of tears and displays an immune modulatory function in corneal fibroblasts [65]. Experiments carried out on a rat model of corneal alkali burns showed that ANG addition in vivo recovered normal cornel transparency and caused a significant reduction in the corneal opacity score compared to the control [66].
A high level of ANG has been measured in wound fluids collected post-injury [67,68]. On the other hand, it has been demonstrated that high levels of ANG in wound fluids are able to induce endothelial cell proliferation and circular angiogenic cell (CAC) differentiation, while the antibody neutralization of ANG in equivalent wound fluids leads to a reduction in their angiogenic properties [67]. Such findings are also supported by further clinical data and prove the positive correlation between ANG and the wound healing process [69].
3. The Role of Copper in Angiogenesis
Copper is an essential element crucial to the health of living organisms [70]. Many studies have highlighted the distinctive biological role of copper ions in both neuronal and endothelial tissues that, despite their different biological structures, share similar signaling pathways [71,72,73]. In the neuronal system, copper ions seem to play a pivotal role in post-synaptic transmission, and its dyshomeostasis is involved in the etiology of numerous neurologic disorders, such as ALS, prion encephalopathies, PD and Alzheimer’s disease [74,75,76,77]. Regarding its interaction with the endothelial system, copper has been recognized as an angiogenic factor. Data from the literature demonstrate that copper ions are able to stimulate endothelial cell migration [78] and neovascularization in avascular rabbit corneas [79], while its depletion, by Cu chelators such as penicillamine and trientine, prevents vessel formation in vivo [80,81]. The role of copper in angiogenesis supports the critical role of the metal in pathological and physiological angiogenic processes, such as cancer and wound repair. Accordingly, increased serum levels of copper have been found in patients with different types of tumors and are related to tumor onset and progression. Based on these findings, copper chelation therapies have been developed for cancer treatment and have proven their efficacy in tumor regression [82].
Later, it was realized that the removal of excess extracellular metal was not in itself a solution [80,81,83]. Copper regulates the expression of different proteins, and in cancer or other pathologies, there are specific metal transporters that are over- or underexpressed. There is a strict balance between pathology and physiology, so the proper and correct restoration of copper homeostasis may represent a valuable pharmacological approach [84].
Indeed, the local modulation of copper’s pro-angiogenic effect provides a promising strategy to enhance tissue repair and regeneration [85] since, as expected, a higher concentration of copper ions (~30 μM) has been detected in the injured site with respect to the peri-wound areas [86].
The mechanism behind the pro-angiogenic activity of copper is the activation and amplification of the angiogenic response, triggered by several cytokines and proteins including VEGF, FGF and ANG, through a multi-faceted action.
The intracellular uptake of copper ions, and their efflux as well as trafficking within the cytoplasmic matrix, is tightly controlled by a complex protein network, since copper shows a reactive nature, which could lead to severe oxidative damage, as long as the free cytosolic copper concentration exceeds the bio-recommended levels (10−18 M) [87]. Copper, indeed, can exist either in the reduced state, Cu+, which shows a high affinity for the thiol and thioether groups of the proteins, or in the oxidized state, Cu2+, which particularly binds to oxygen atoms and imidazole nitrogen. Although its double nature allows copper to interact with several proteins, thus controlling numerous biochemical processes, the passage between the two states, Cu+ and Cu2+, can generate hydroxyl radicals [88].
Copper enters the cell through the copper transporters 1 and 2 (CTR1 and CTR2), which are membrane proteins with a channel-like structure, widely present in several cell types and tissues [89,90,91]. Before its transfer, to ensure efficient transport across the cell membrane, Cu2+ is reduced to Cu+, by membrane metal reductases [92], and within the cell, copper’s distribution to mitochondrial, nuclear and vesicular targets is mediated by several metal chaperones, namely glutathione (GSH), Menkes protein, copper chaperone for superoxide dismutase (SOD) and antioxidant-1 (ATOX-1) [93].
During the early stages of angiogenesis, intracellular copper has been demonstrated to stabilize the hypoxia inducible factor-1 (HIF-1) structure, thereby promoting its transcriptional activity on angiogenic genes including VEGF and ceruloplasmin genes [94,95,96]. Ceruloplasmin, indeed, is a serum globulin protein that, by binding to copper ions, stimulates neovascularization, whereas its copper-deprived form is unable to induce blood vessel formation [97]. Regarding VEGF, experiments on cultured human cardiomyocytes showed that copper ions, at a concentration of 5 μM, stimulate insulin-like growth factor-1 (IGF-1)-induced VEGF expression [98]. Moreover, copper ions have been found to promote vasodilation by activating the endothelial nitric oxide synthase enzyme (eNOS) and nitric oxide (NO) release. In this regard, several studies suggest that extracellular Cu2+ promotes transmembrane calcium ion influx, increasing the concentration of intracellular Ca2+ ions, which, via the calcium-calmodulin pathway, enhances the eNOS activity [99]. On the other hand, intracellular copper, as a cofactor of the cuproenzyme SOD, protects NO from superoxide anion scavenging, thus increasing its half-life [100]. Furthermore, copper complexes of the fibroblast growth factor 1 (FGF-1) and the lysyl oxidase (LOX) [101] stimulate FGF secretion [102] and ECM degradation, respectively, thus promoting cell migration and proliferation. It is important to note that the copper-induced angiogenic effects are highly cell-type-specific, since it has been demonstrated that copper ions are able to enhance the proliferation of endothelial cells, while, under the same conditions, no stimulating effects have been found in arterial smooth muscle cells and even weaker effects have been measured in cultured fibroblasts [103].
4. Copper Modulates Angiogenin Activity
Copper ions amplify and promote vascular permeabilization as well as endothelial cells’ migration and proliferation by binding to several factors involved in the angiogenic process. Among the pro-angiogenic effectors, it has been found that copper controls and modulates the angiogenic response and the biological function of ANG.
Copper increases ANG expression in the HUVEC cell line, so it is possible that the increase in extracellular copper during the angiogenesis process may regulate the angiogenin level [26].
Indeed, copper is the only metal mobilized during the angiogenesis process from the intra- to extracellular space [104]. ANG is a secreted protein that translocates inside the cell, so a direct interaction between metal and protein may constitute a system of mutual control between the two components in different angiogenic steps.
Previous works reported that the complex formation between divalent copper and ANG decreases both the nuclear translocation of the protein and its ribonucleolytic activity. Moreover, the interaction between ANG and calf pulmonary artery endothelial cells increases 4.3-fold in the presence of copper ions [105].
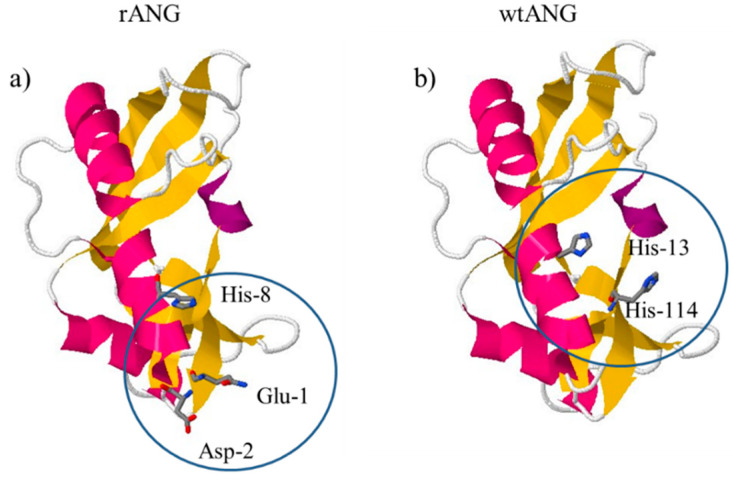
The ANG–copper complex formation, the metal coordination environment as well as the copper-induced effect on ANG mostly depend on the chemical structure of the protein, and angiogenin, in particular, shows two different forms, the recombinant and the wild type. The recombinant form of ANG (rANG), expressed in bacterial vectors and typically used for research works, contains an extra methionine residue at the N-terminal domain. Differently, the wild-type angiogenin (wtANG), physiologically present in human plasma, shows glutamine as the first residue, which is spontaneously cyclized to a pyroglutamate ring. A recent research work showed that rANG and wtANG share a similar secondary structure rich in β-strands, but with different metal binding. Circular dichroism (CD) and electrospray ionization mass spectrometry (ESI-MS) experiments, indeed, suggest a 2:1 metal to ligand stoichiometry of the metal–protein complex for rANG, while a 1:1 metal to ligand stoichiometry for the copper complex of wtANG, at physiological pH [24], has been noted. Furthermore, spectroscopic data revealed a strong ligand field around the metal core of the rANG–Cu(II) complex, which involves four nitrogen donors in a planar arrangement [24,106]. Meanwhile, a low ligand field seems to characterize the wtANG–Cu(II) complex, which most likely involves two imidazole nitrogen atoms, one deprotonated nitrogen and one oxygen atom [24,106]. NMR measurements allowed the identification, at physiological pH, of the N-terminal group of the methionine, the deprotonated amide nitrogen of Glu-1 and Asp-2 and the imidazole nitrogen of the His-8 residues in the coordination environment of the rANG–Cu(II) complex. On the other hand, His-114 and His-13, which also form the catalytic site of ANG, are the metal anchoring sites for the formation of the wtANG–Cu(II) complex (Figure 6). Accordingly, copper ions more efficiently influence the physiological form of ANG, with respect to the recombinant angiogenin, since the metal–protein complex involves its catalytic site. In this regard, in vitro experiments of capillary-like tube formation and the RNase enzymatic assay evidenced that divalent copper decreases the activity of both the proteins, but a higher concentration of Cu2+ was required for the rANG sample to reach the same decrease in both tube formation and enzymatic action observed for wtANG [24].
Figure 6.
Putative anchor site of Cu2+ in (a) rANG and (b) wtANG copper complexes (PDB ID 1ANG).
The design of peptides able to mimic the functional sites of proteins is an interesting strategy aimed at understanding protein activity and cofactor binding as metal ions as well as in developing new potential drugs [107,108,109]. Peptides encompassing the N-terminal residues 1–17 of the protein, Ang(1–17), with the amino free, and AcAng(1–17), the analogous form with the N-terminal amino group acetylated, were synthesized to highlight the role of the amino group in copper binding [110]. A physiological concentration of copper increases actin staining, an effect counteracted by the addition of copper complexes formed by N-terminal peptides. The comparison between wtANG, rANG and N-terminal peptides highlights that a different copper coordination environment affects its biological activities.
The medical treatment of pathological angiogenesis as well as the modulation of physiological vessel growth, through the use of angiogenin or its peptide fragments, can be improved by using nanocarriers. Experiments carried out on endothelial cells revealed a significant improvement in wound closure and angiogenic activity after treatment with a multifunctional platform obtained by anchoring ANG to the surfaces of AuNPs [111]. Notably, this new hybrid nanoplatform triggers intracellular copper trafficking, confirming the correlation between metal and ANG in the angiogenic process.
5. Conclusions
Angiogenin is a secreted protein that regulates different angiogenesis steps by means of multiple pathways, many of which are yet to be fully elucidated. Some of these pathways are also activated by copper, another angiogenic factor. Copper regulates ANG expression in endothelial cells and many experimental studies suggest that metal drives ANG intracellular localization. On the other hand, experiments carried out on cancer cells show that ANG may regulate intracellular copper levels. Therefore, ANG and copper activity in angiogenic processes appear to be closely related and the understanding of their common biochemical pathways is expected to pave the way to new pharmaceutical applications in wound healing.
Acknowledgments
T.M., C.S. and D.L.M. acknowledge the Consorzio Interuniversitario di Ricerca in Chimica dei Metalli nei Sistemi Biologici (C.I.R.C.M.S.B.), Bari, Italy.
Author Contributions
Conceptualization, C.S. and D.L.M.; writing—original draft preparation, L.M.C., C.S. and D.L.M.; writing—review and editing, T.M., C.S., D.L.M.; supervision, D.L.M.; funding acquisition, C.S., T.M. and D.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian ministry of Education, Universities and Research (MIUR) (PRIN call, project code: 2017WBZFHL), the ERA-NET Cofund “M-ERA-NET 2” call (Project name “SmartHyCAR”, number: 4274), the University of Catania (PIAno di inCEntivi per la RIcerca di Ateneo 2020/2022 CHANCE_Linea di Intervento 1 e GRABIO_Linea di intervento 2), Beneficentia Stiftung, Vaduz (BEN2019/48), the University of Pisa, “PRA—Progetti di Ricerca di Ateneo” Institutional Research Grants—Project no. PRA_2020_58 “Agenti innovative e nanosistemi per target molecolari nell’ambito dell’oncologia di precisione”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reinke J.M., Sorg H. Wound Repair and Regeneration. Eur. Surg. Res. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 2.Broughton G., 2nd, Janis J.E., Attinger C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006;117:12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 3.Sekhon U.D.S., Sen Gupta A. Platelets and platelet-inspired biomaterials technologies in wound healing applications. ACS Biomater. Sci. Eng. 2018;4:1176–1192. doi: 10.1021/acsbiomaterials.7b00013. [DOI] [PubMed] [Google Scholar]
- 4.Hesketh M., Sahin K.B., West Z.E., Murray R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017;18:1545. doi: 10.3390/ijms18071545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stojadinovic O., Yin N., Lehmann J., Pastar I., Kirsner R.S., Tomic-Canic M. Increased number of Langerhans cells in the epidermis of diabetic foot ulcers correlates with healing outcome. Immunol. Res. 2013;57:222–228. doi: 10.1007/s12026-013-8474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., Wu J., Luo G., He W. Functions of Vγ4 T cells and dendritic epidermal T cells on skin wound healing. Front. Immunol. 2018;9:1099. doi: 10.3389/fimmu.2018.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jameson J., Ugarte K., Chen N., Yachi P., Fuchs E., Boismenu R., Havran W.L. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 8.Gushiken L.F.S., Beserra F.P., Bastos J.K., Jackson C.J., Pellizzon C.H. Cutaneous wound healing: An update from physiopathology to current therapies. Life. 2021;11:665. doi: 10.3390/life11070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velnar T., Bailey T., Smrkolj V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 10.Khoury C.C., Ziyadeh F.N. Angiogenic factors. Contrib. Nephrol. 2011;170:83–92. doi: 10.1159/000324950. [DOI] [PubMed] [Google Scholar]
- 11.Shibuya M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 13.Vincent L., Rafii S. Vascular frontiers without borders: Multifaceted roles of platelet-derived growth factor (PDGF) in supporting postnatal angiogenesis and lymphangiogenesis. Cancer Cell. 2004;6:307–309. doi: 10.1016/j.ccr.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Menon S.N., Flegg J.A., McCue S.W., Schugart R.C., Dawson R.A., McElwain D.L. Modelling the interaction of keratinocytes and fibroblasts during normal and abnormal wound healing processes. Proc. R. Soc. B Biol. Sci. 2012;279:3329–3338. doi: 10.1098/rspb.2012.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demidova-Rice T.N., Hamblin M.R., Herman I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Ski. Wound Care. 2012;25:304–314. doi: 10.1097/01.ASW.0000416006.55218.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo S., Dipietro L.A. Factors affecting wound healing. J. Dent. Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsaie M.L. Update on management of keloid and hypertrophic scars: A systemic review. J. Cosmet. Dermatol. 2021;20:2729–2738. doi: 10.1111/jocd.14310. [DOI] [PubMed] [Google Scholar]
- 18.Limandjaja G.C., Niessen F.B., Scheper R.J., Gibbs S. The keloid disorder: Heterogeneity, histopathology, mechanisms and models. Front. Cell. Dev. Biol. 2020;8:360. doi: 10.3389/fcell.2020.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mokos Z.B., Jović A., Grgurević L., Dumić-Čule I., Kostović K., Čeović R., Marinović B. Current therapeutic approach to hypertrophic scars. Front. Med. 2017;4:83. doi: 10.3389/fmed.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limandjaja G.C., Niessen F.B., Scheper R.J., Gibbs S. Hypertrophic scars and keloids: Overview of the evidence and practical guide for differentiating between these abnormal scars. Exp. Dermatol. 2021;30:146–161. doi: 10.1111/exd.14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong D.G., Swerdlow M.A., Armstrong A.A., Conte M.S., Padula W.V., Bus S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot Ankle Res. 2020;13:16. doi: 10.1186/s13047-020-00383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng J., Xu Z. Three decades of research on angiogenin: A review and perspective. Acta Biochim. Biophys. Sin. 2016;48:399–410. doi: 10.1093/abbs/gmv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noschka R., Gerbl F., Löffler F., Kubis J., Rodríguez A.A., Mayer D., Grieshober M., Holch A., Raasholm M., Forssmann W.G., et al. Unbiased identification of angiogenin as an endogenous antimicrobial protein with activity against virulent Mycobacterium tuberculosis. Front. Microbiol. 2021;11:618278. doi: 10.3389/fmicb.2020.618278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Mendola D., Arnesano F., Hansson Ö., Giacomelli C., Calò V., Mangini V., Magrì A., Bellia F., Trincavelli M.L., Martini C., et al. Copper binding to naturally occurring, lactam form of angiogenin differs from that to recombinant protein, affecting their activity. Metallomics. 2016;8:118–124. doi: 10.1039/C5MT00216H. [DOI] [PubMed] [Google Scholar]
- 25.Sen C.K., Khanna S., Venojarvi M., Trikha P., Ellison E.C., Hunt T.K., Roy S. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1821–H1827. doi: 10.1152/ajpheart.01015.2001. [DOI] [PubMed] [Google Scholar]
- 26.Giacomelli C., Trincavelli M.L., Satriano C., Hansson Ö., La Mendola D., Rizzarelli E., Martini C. Copper (II) ions modulate Angiogenin activity in human endothelial cells. Int. J. Biochem. Cell. Biol. 2015;60:185–196. doi: 10.1016/j.biocel.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro R., Strydom D.J., Olson K.A., Vallee B.L. Isolation of angiogenin from normal human plasma. Biochemistry. 1987;26:5141–5146. doi: 10.1021/bi00390a037. [DOI] [PubMed] [Google Scholar]
- 28.Strydom D.J., Fett J.W., Lobb R.R., Alderman E.M., Bethune J.L., Riordan J.F., Vallee B.L. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985;24:5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- 29.Thiyagarajan N., Acharya K.R. Crystal structure of human angiogenin with an engineered loop exhibits conformational flexibility at the functional regions of the molecule. FEBS Open Bio. 2012;3:65–70. doi: 10.1016/j.fob.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russo N., Shapiro R., Acharya K.R., Riordan J.F., Vallee B.L. Role of glutamine-117 in the ribonucleolytic activity of human angiogenin. Proc. Natl. Acad. Sci. USA. 1994;91:2920–2924. doi: 10.1073/pnas.91.8.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo N., Nobile V., Di Donato A., Riordan J.F., Vallee B.L. The C-terminal region of human angiogenin has a dual role in enzymatic activity. Proc. Natl. Acad. Sci. USA. 1996;93:3243–3247. doi: 10.1073/pnas.93.8.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu G.F., Chang S.I., Riordan J.F., Vallee B.L. An angiogenin-binding protein from endothelial cells. Proc. Natl. Acad. Sci. USA. 1991;88:2227–2231. doi: 10.1073/pnas.88.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moroianu J., Riordan J.F. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proc. Natl. Acad. Sci. USA. 1994;91:1677–1681. doi: 10.1073/pnas.91.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kishimoto K., Liu S., Tsuji T., Olson K.A., Hu G.F. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene. 2005;24:445–456. doi: 10.1038/sj.onc.1208223. [DOI] [PubMed] [Google Scholar]
- 35.Hu G.F., Riordan J.F., Vallee B.L. A putative angiogenin receptor in angiogenin-responsive human endothelial cells. Proc. Natl. Acad. Sci. USA. 1997;94:2204–2209. doi: 10.1073/pnas.94.6.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu W., Goncalves K.A., Li S., Kishikawa H., Sun G., Yang H., Vanli N., Wu Y., Jiang Y., Hu M.G., et al. Plexin-B2 mediates physiologic and pathologic functions of angiogenin. Cell. 2017;171:849–864. doi: 10.1016/j.cell.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S., Yu D., Xu Z.P., Riordan J.F., Hu G.F. Angiogenin activates Erk1/2 in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2001;287:305–310. doi: 10.1006/bbrc.2001.5568. [DOI] [PubMed] [Google Scholar]
- 38.Ibaragi S., Yoshioka N., Kishikawa H., Hu J.K., Sadow P.M., Li M., Hu G.F. Angiogenin-stimulated rRNA transcription is essential for initiation and survival of AKT-induced prostate intraepithelial neoplasia. Mol. Cancer Res. 2009;7:415–424. doi: 10.1158/1541-7786.MCR-08-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Z., Monti D.M., Hu G.F. Angiogenin activates human umbilical artery smooth muscle cells. Biochem. Biophys. Res. Commun. 2001;285:909–914. doi: 10.1006/bbrc.2001.5255. [DOI] [PubMed] [Google Scholar]
- 40.Trouillon R., Kang D.K., Park H., Chang S.I., O’Hare D. Angiogenin induces nitric oxide synthesis in endothelial cells through PI-3 and Akt kinases. Biochemistry. 2010;49:3282–3288. doi: 10.1021/bi902122w. [DOI] [PubMed] [Google Scholar]
- 41.Sheng J., Yu W., Gao X., Xu Z., Hu G.F. Angiogenin stimulates ribosomal RNA transcription by epigenetic activation of the ribosomal DNA promoter. J. Cell. Physiol. 2014;229:521–529. doi: 10.1002/jcp.24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivanov P., Emara M.M., Villen J., Gygi S.P., Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell. 2011;43:613–623. doi: 10.1016/j.molcel.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elkordy A., Mishima E., Niizuma K., Akiyama Y., Fujimura M., Tominaga T., Abe T. Stress-induced tRNA cleavage and tiRNA generation in rat neuronal PC12 cells. J. Neurochem. 2018;146:560–569. doi: 10.1111/jnc.14321. [DOI] [PubMed] [Google Scholar]
- 44.Pyatibratov M.G., Kostyukova A.S. New insights into the role of angiogenin in actin polymerization. Int. Rev. Cell. Mol. Biol. 2012;295:175–198. doi: 10.1016/B978-0-12-394306-4.00011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satriano C., Munzone A., Cucci L.M., Giacomelli C., Trincavelli M.L., Martini C., Rizzarelli E., La Mendola D. Angiogenin-mimetic peptide functionalised gold nanoparticles for cancer therapy applications. Microchem. J. 2018;136:157–163. doi: 10.1016/j.microc.2016.09.016. [DOI] [Google Scholar]
- 46.Hu G.F., Riordan J.F. Angiogenin enhances actin acceleration of plasminogen activation. Biochem. Biophys. Res. Commun. 1993;197:682–687. doi: 10.1006/bbrc.1993.2533. [DOI] [PubMed] [Google Scholar]
- 47.Weisel J.W. Fibrinogen and fibrin. Adv. Protein Chem. 2005;70:247–299. doi: 10.1016/S0065-3233(05)70008-5. [DOI] [PubMed] [Google Scholar]
- 48.Bläser J., Triebel S., Kopp C., Tschesche H. A highly sensitive immunoenzymometric assay for the determination of angiogenin. Eur. J. Clin. Chem. Clin. Biochem. 1993;31:513–516. doi: 10.1515/cclm.1993.31.8.513. [DOI] [PubMed] [Google Scholar]
- 49.Yu D., Sun J., Weng Y., Luo L., Sheng J., Xu Z. Serum angiogenin as a potential biomarker for early detection of colorectal adenomas and colorectal cancer. Anticancer Drugs. 2021;32:703–708. doi: 10.1097/CAD.0000000000001047. [DOI] [PubMed] [Google Scholar]
- 50.Madazli R., Atiş A., Uzun H., Aksu F. Mid-trimester amniotic fluid angiogenin, lactate dehydrogenase and fibronectin in the prediction of preterm delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003;106:160–164. doi: 10.1016/S0301-2115(02)00264-6. [DOI] [PubMed] [Google Scholar]
- 51.Morelli C., Tiloca C., Colombrita C., Zambon A., Soranna D., Lafronza A., Solca F., Carelli L., Poletti B., Doretti A., et al. CSF angiogenin levels in amyotrophic lateral Sclerosis-Frontotemporal dementia spectrum. Amyotroph. Lateral Scler. Front. Degener. 2020;21:63–69. doi: 10.1080/21678421.2019.1704016. [DOI] [PubMed] [Google Scholar]
- 52.Ivanov P., O’Day E., Emara M.M., Wagner G., Lieberman J., Anderson P. G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc. Natl. Acad. Sci. USA. 2014;111:18201–18206. doi: 10.1073/pnas.1407361111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee S.H., Kim K.W., Min K.M., Kim K.W., Chang S.I., Kim J.C. Angiogenin reduces immune inflammation via inhibition of TANK-binding kinase 1 expression in human corneal fibroblast cells. Mediators Inflamm. 2014;2014:861435. doi: 10.1155/2014/861435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eleftheriadis T., Pissas G., Sounidaki M., Antoniadis N., Antoniadi G., Liakopoulos V., Stefanidis I. Angiogenin is upregulated during the alloreactive immune response and has no effect on the T-cell expansion phase, whereas it affects the contraction phase by inhibiting CD4+ T-cell apoptosis. Exp. Ther. Med. 2016;12:3471–3475. doi: 10.3892/etm.2016.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hooper L.V., Stappenbeck T.S., Hong C.V., Gordon J.I. Angiogenins: A new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 56.Rajashekhar G., Loganath A., Roy A.C., Wong Y.C. Expression and localization of angiogenin in placenta: Enhanced levels at term over first trimester villi. Mol. Reprod. Dev. 2002;62:159–166. doi: 10.1002/mrd.10116. [DOI] [PubMed] [Google Scholar]
- 57.Greenway M.J., Andersen P.M., Russ C., Ennis S., Cashman S., Donaghy C., Patterson V., Swingler R., Kieran D., Prehn J., et al. ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat. Genet. 2006;38:411–413. doi: 10.1038/ng1742. [DOI] [PubMed] [Google Scholar]
- 58.Bradshaw W.J., Rehman S., Pham T.T., Thiyagarajan N., Lee R.L., Subramanian V., Acharya K.R. Structural insights into human angiogenin variants implicated in Parkinson’s disease and Amyotrophic Lateral Sclerosis. Sci. Rep. 2017;7:41996. doi: 10.1038/srep41996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koutroubakis I.E., Xidakis C., Karmiris K., Sfiridaki A., Kandidaki E., Kouroumalis E.A. Serum angiogenin in inflammatory bowel disease. Dig. Dis. Sci. 2004;49:1758–1762. doi: 10.1007/s10620-004-9565-4. [DOI] [PubMed] [Google Scholar]
- 60.Rogov I.A., Tsuman V.G., Mashkov A.E., Plaksina G.V., Pykhteev D.A., Kirimov R.Y., Shalygina A.M., Tikhomirova N.A., Komolova G.S. Method of Treatment of Wounds and Ulcerous Damages. RU2197263C1. Patent. 2003 January 27;
- 61.Yurina N.V., Ageeva T.A., Goryachkin A.M., Varaksin N.A., Ryabicheva T.G., Ostanin A.A., Chernykh E.R., Romashchenko A.V., Proskurina A.S., Bogachev S., et al. Effects of recombinant angiogenin on collagen fiber formation and angiogenesis in the dermis of wistar rats. Clin. Cosmet. Investig. Dermatol. 2021;14:187–196. doi: 10.2147/CCID.S294825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bourne W.M. Biology of the corneal endothelium in health and disease. Eye. 2003;17:912–918. doi: 10.1038/sj.eye.6700559. [DOI] [PubMed] [Google Scholar]
- 63.Kim K.W., Park S.H., Lee S.J., Kim J.C. Ribonuclease 5 facilitates corneal endothelial wound healing via activation of PI3-kinase/Akt pathway. Sci. Rep. 2016;6:31162. doi: 10.1038/srep31162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim K.W., Lee S.J., Park S.H., Kim J.C. Ex Vivo Functionality of 3D Bioprinted Corneal Endothelium Engineered with Ribonuclease 5-Overexpressing Human Corneal Endothelial Cells. Adv. Healthc. Mater. 2018;7:e1800398. doi: 10.1002/adhm.201800398. [DOI] [PubMed] [Google Scholar]
- 65.Vinekar A., Nair A.P., Sinha S., Vaidya T., Chakrabarty K., Shetty R., Ghosh A., Sethu S. Tear Fluid Angiogenic Factors: Potential Noninvasive Biomarkers for Retinopathy of Prematurity Screening in Preterm Infants. Investig. Ophthalmol. Vis. Sci. 2021;62:2. doi: 10.1167/iovs.62.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee S.H., Kim K.W., Joo K., Kim J.C. Angiogenin ameliorates corneal opacity and neovascularization via regulating immune response in corneal fibroblasts. BMC Ophthalmol. 2016;16:57. doi: 10.1186/s12886-016-0235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan S.C., Wu L.W., Chen C.L., Shieh S.J., Chiu H.Y. Angiogenin expression in burn blister fluid: Implications for its role in burn wound neovascularization. Wound Repair Regen. 2012;20:731–739. doi: 10.1111/j.1524-475X.2012.00819.x. [DOI] [PubMed] [Google Scholar]
- 68.Pan S.C., Tsai Y.H., Chuang C.C., Cheng C.M. Preliminary assessment of burn depth by paper-based ELISA for the detection of angiogenin in burn blister fluid-a proof of concept. Diagnostics. 2020;10:127. doi: 10.3390/diagnostics10030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pan S.C., Lee C.H., Chen C.L., Fang W.Y., Wu L.W. Angiogenin attenuates scar formation in burn patients by reducing fibroblast proliferation and transforming growth factor β1 secretion. Ann. Plast. Surg. 2018;80:S79–S83. doi: 10.1097/SAP.0000000000001306. [DOI] [PubMed] [Google Scholar]
- 70.Festa R.A., Thiele D.J. Copper: An essential metal in biology. Curr. Biol. 2011;21:R877–R883. doi: 10.1016/j.cub.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grubman A., White A.R. Copper as a key regulator of cell signalling pathways. Expert Rev. Mol. Med. 2014;16:e11. doi: 10.1017/erm.2014.11. [DOI] [PubMed] [Google Scholar]
- 72.Bharathi Devi S.R., Dhivya M.A., Sulochana K.N. Copper transporters and chaperones: Their function on angiogenesis and cellular signalling. J. Biosci. 2016;41:487–496. doi: 10.1007/s12038-016-9629-6. [DOI] [PubMed] [Google Scholar]
- 73.La Mendola D. Nerve growth factor catches copper in neuronal inning. Neural Regen. Res. 2020;15:665–666. doi: 10.4103/1673-5374.266912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urso E., Maffia M. Behind the link between copper and angiogenesis: Established mechanisms and an overview on the role of vascular copper transport systems. J. Vasc. Res. 2015;52:172–196. doi: 10.1159/000438485. [DOI] [PubMed] [Google Scholar]
- 75.Magrì A., Tabbì G., Cucci L.M., Satriano C., Pietropaolo A., Malgieri G., Isernia C., La Mendola D. The curious case of opossum prion: A physicochemical study on copper(ii) binding to the bis-decarepeat fragment from the protein N-terminal domain. Dalton Trans. 2019;48:17533–17543. doi: 10.1039/C9DT02510C. [DOI] [PubMed] [Google Scholar]
- 76.Gromadzka G., Tarnacka B., Flaga A., Adamczyk A. Copper Dyshomeostasis in Neurodegenerative Diseases-Therapeutic Implications. Int. J. Mol. Sci. 2020;21:9259. doi: 10.3390/ijms21239259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.La Mendola D., Arena G., Pietropaolo A., Satriano C., Rizzarelli E. Metal ion coordination in peptide fragments of neurotrophins: A crucial step for understanding the role and signaling of these proteins in the brain. Coord. Chem. Rev. 2021;435:213790. doi: 10.1016/j.ccr.2021.213790. [DOI] [Google Scholar]
- 78.McAuslan B.R., Reilly W. Endothelial cell phagokinesis in response to specific metal ions. Exp. Cell. Res. 1980;130:147–157. doi: 10.1016/0014-4827(80)90051-8. [DOI] [PubMed] [Google Scholar]
- 79.Ziche M., Jones J., Gullino P.M. Role of prostaglandin E1 and copper in angiogenesis. J. Natl. Cancer Inst. 1982;69:475–482. [PubMed] [Google Scholar]
- 80.Brem S., Tsanaclis A.M., Zagzag D. Anticopper treatment inhibits pseudopodial protrusion and the invasive spread of 9L gliosarcoma cells in the rat brain. Neurosurgery. 1990;26:391–396. doi: 10.1227/00006123-199003000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Denoyer D., Masaldan S., La Fontaine S., Cater M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics. 2015;7:1459–1476. doi: 10.1039/C5MT00149H. [DOI] [PubMed] [Google Scholar]
- 82.Denoyer D., Clatworthy S.A.S., Cater M.A. Copper complexes in cancer therapy. Met. Ions Life Sci. 2018:18. doi: 10.1515/9783110470734-022. [DOI] [PubMed] [Google Scholar]
- 83.La Mendola D., Rizzarelli E. Perspectives in medicinal chemistry: Metallomics and new targets in metal-based drug discovery. Curr. Top. Med. Chem. 2016;16:3381–3382. doi: 10.2174/156802661629161020215241. [DOI] [PubMed] [Google Scholar]
- 84.La Mendola D., Giacomelli C., Rizzarelli E. Intracellular bioinorganic chemistry and cross talk among different-omics. Curr. Top. Med. Chem. 2016;16:3103–3130. doi: 10.2174/1568026616666160715164212. [DOI] [PubMed] [Google Scholar]
- 85.Mirastschijski U., Martin A., Jorgensen L.N., Sampson B., Ågren M.S. Zinc, copper, and selenium tissue levels and their relation to subcutaneous abscess, minor surgery, and wound healing in humans. Biol. Trace Elem. Res. 2013;153:76–83. doi: 10.1007/s12011-013-9658-z. [DOI] [PubMed] [Google Scholar]
- 86.Cangul I.T., Gul N.Y., Topal A., Yilmaz R. Evaluation of the effects of topical tripeptide-copper complex and zinc oxide on open-wound healing in rabbits. Vet. Dermatol. 2006;17:417–423. doi: 10.1111/j.1365-3164.2006.00551.x. [DOI] [PubMed] [Google Scholar]
- 87.Rae T.D., Schmidt P.J., Pufahl R.A., Culotta V.C., O’Halloran T.V. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 88.Husain N., Mahmood R. Copper(II) generates ROS and RNS, impairs antioxidant system and damages membrane and DNA in human blood cells. Environ. Sci. Pollut. Res. Int. 2019;26:20654–20668. doi: 10.1007/s11356-019-05345-1. [DOI] [PubMed] [Google Scholar]
- 89.Magistrato A., Pavlin M., Qasem Z., Ruthstein S. Copper trafficking in eukaryotic systems: Current knowledge from experimental and computational efforts. Curr. Opin. Struct. Biol. 2019;58:26–33. doi: 10.1016/j.sbi.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maung M.T., Carlson A., Olea-Flores M., Elkhadragy L., Schachtschneider K.M., Navarro-Tito N., Padilla-Benavides T. The molecular and cellular basis of copper dysregulation and its relationship with human pathologies. FASEB J. 2021;35:e21810. doi: 10.1096/fj.202100273RR. [DOI] [PubMed] [Google Scholar]
- 91.Lutsenko S. Copper trafficking to the secretory pathway. Metallomics. 2016;8:840–852. doi: 10.1039/C6MT00176A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kidane T.Z., Farhad R., Lee K.J., Santos A., Russo E., Linder M.C. Uptake of copper from plasma proteins in cells where expression of CTR1 has been modulated. Biometals. 2012;25:697–709. doi: 10.1007/s10534-012-9528-8. [DOI] [PubMed] [Google Scholar]
- 93.O’Halloran T.V., Culotta V.C. Metallochaperones, an intracellular shuttle service for metal ions. J. Biol. Chem. 2000;275:25057–25060. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- 94.Fu H., Ding X., Zhang W., Kang Y.J. Profiling of nuclear copper-binding proteins under hypoxic condition. Biometals. 2019;32:329–341. doi: 10.1007/s10534-019-00171-x. [DOI] [PubMed] [Google Scholar]
- 95.Wu Z., Zhang W., Kang Y.J. Copper affects the binding of HIF-1α to the critical motifs of its target genes. Metallomics. 2019;11:429–438. doi: 10.1039/C8MT00280K. [DOI] [PubMed] [Google Scholar]
- 96.Liu X., Zhang W., Wu Z., Yang Y., Kang Y.J. Copper levels affect targeting of hypoxia-inducible factor 1α to the promoters of hypoxia-regulated genes. J. Biol. Chem. 2018;293:14669–14677. doi: 10.1074/jbc.RA118.001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Finney L., Vogt S., Fukai T., Glesne D. Copper and angiogenesis: Unravelling a relationship key to cancer progression. Clin. Exp. Pharmacol. Physiol. 2009;36:88–94. doi: 10.1111/j.1440-1681.2008.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang Y., Reynolds C., Xiao C., Feng W., Zhou Z., Rodriguez W., Tyagi S.C., Eaton J.W., Saari J.T., Kang Y.J. Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J. Exp. Med. 2007;204:657–666. doi: 10.1084/jem.20061943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Demura Y., Ishizaki T., Ameshima S., Okamura S., Hayashi T., Matsukawa S., Miyamori I. The activation of nitric oxide synthase by copper ion is mediated by intracellular Ca2+ mobilization in human pulmonary arterial endothelial cells. Br. J. Pharmacol. 1998;125:1180–1187. doi: 10.1038/sj.bjp.0702197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kishimoto T., Oguri T., Ueda D., Tada M. Copper enhances EDNO (endothelium-derived nitric oxide) activity by cultured human vascular endothelial cells. Hum. Cell. 1996;9:117–124. [PubMed] [Google Scholar]
- 101.Shanbhag V., Jasmer-McDonald K., Zhu S., Martin A.L., Gudekar N., Khan A., Ladomersky E., Singh K., Weisman G.A., Petris M.J. ATP7A delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc. Natl. Acad. Sci. USA. 2019;116:6836–6841. doi: 10.1073/pnas.1817473116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Landriscina M., Bagalá C., Mandinova A., Soldi R., Micucci I., Bellum S., Prudovsky I., Maciag T. Copper induces the assembly of a multiprotein aggregate implicated in the release of fibroblast growth factor 1 in response to stress. J. Biol. Chem. 2001;276:25549–25557. doi: 10.1074/jbc.M102925200. [DOI] [PubMed] [Google Scholar]
- 103.Hu G.F. Copper stimulates proliferation of human endothelial cells under culture. J. Cell. Biochem. 1998;69:326–335. doi: 10.1002/(SICI)1097-4644(19980601)69:3<326::AID-JCB10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 104.Finney L., Mandava S., Ursos L., Zhang W., Rodi D., Vogt S., Legnini D., Maser J., Ikpatt F., Olopade O.I., et al. X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc. Natl. Acad. Sci. USA. 2007;104:2247–2252. doi: 10.1073/pnas.0607238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Soncin F., Guitton J.D., Cartwright T., Badet J. Interaction of human angiogenin with copper modulates angiogenin binding to endothelial cells. Biochem. Biophys. Res. Commun. 1997;236:604–610. doi: 10.1006/bbrc.1997.7018. [DOI] [PubMed] [Google Scholar]
- 106.La Mendola D., Farkas D., Bellia F., Magrì A., Travaglia A., Hansson Ö., Rizzarelli E. Probing the copper(II) binding features of angiogenin. Similarities and differences between a N-terminus peptide fragment and the recombinant human protein. Inorg. Chem. 2012;51:128–141. doi: 10.1021/ic201300e. [DOI] [PubMed] [Google Scholar]
- 107.La Mendola D., Magrì A., Campagna T., Campitiello M.A., Raiola L., Isernia C., Hansson O., Bonomo R.P., Rizzarelli E. A doppel alpha-helix peptide fragment mimics the copper(II) interactions with the whole protein. Chem. Eur. J. 2010;16:6212–6223. doi: 10.1002/chem.200902405. [DOI] [PubMed] [Google Scholar]
- 108.Groß A., Hashimoto C., Sticht H., Eichler J. Synthetic Peptides as Protein Mimics. Front. Bioeng. Biotechnol. 2016;3:211. doi: 10.3389/fbioe.2015.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Henninot A., Collins J.C., Nuss J.M. The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018;61:1382–1414. doi: 10.1021/acs.jmedchem.7b00318. [DOI] [PubMed] [Google Scholar]
- 110.Magrì A., Munzone A., Peana M., Medici S., Zoroddu M.A., Hansson O., Satriano C., Rizzarelli E., La Mendola D. Coordination environment of Cu(II) ions bound to N-terminal peptide fragments of angiogenin protein. Int. J. Mol. Sci. 2016;17:1240. doi: 10.3390/ijms17081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cucci L.M., Trapani G., Hansson Ö., La Mendola D., Satriano C. Gold nanoparticles functionalized with angiogenin for wound care application. Nanomaterials. 2021;11:201. doi: 10.3390/nano11010201. [DOI] [PMC free article] [PubMed] [Google Scholar]