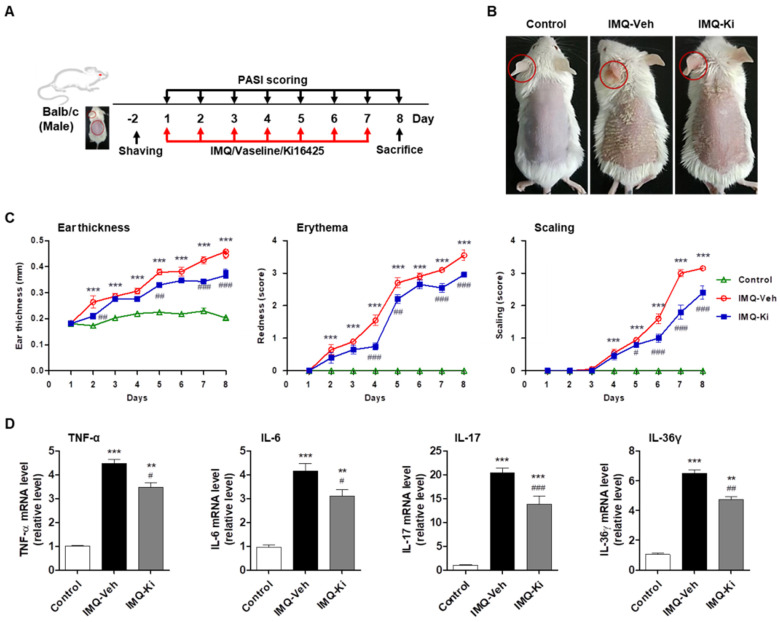
Figure 1.
Ki16425 ameliorates skin symptoms and inflammation in imiquimod (IMQ)-induced psoriasis-like mice. (A) Experimental design of the IMQ-induced psoriasis-like mouse model and ki16425 treatment. (B) IMQ cream or Vaseline was topically applied daily to 7-week-old Balb/c mice for 7 days. Then, representative photographs of back skin were captured, and the phenotypical symptoms of the mouse back skin were observed. The macroscopic appearance is shown. Control, Vaseline-applied normal control; IMQ-Veh, IMQ with vehicle-treated group; IMQ-Ki, IMQ with ki16425-treated group. (C) Ear thickness and Psoriasis Area Severity Index (PASI) scores (redness and scaling) were evaluated during the experimental periods (n = 9/group). (D) The mRNA expression levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-17, and IL-36γ in skin from experimental mice were analyzed by quantitative real-time RT-PCR (qRT-PCR) (n = 4/group). The data are represented as the mean ± standard error of the mean (SEM). ** p < 0.01, *** p < 0.005 vs. Control; # p < 0.05, ## p < 0.01, ### p < 0.005 vs. IMQ-Veh.