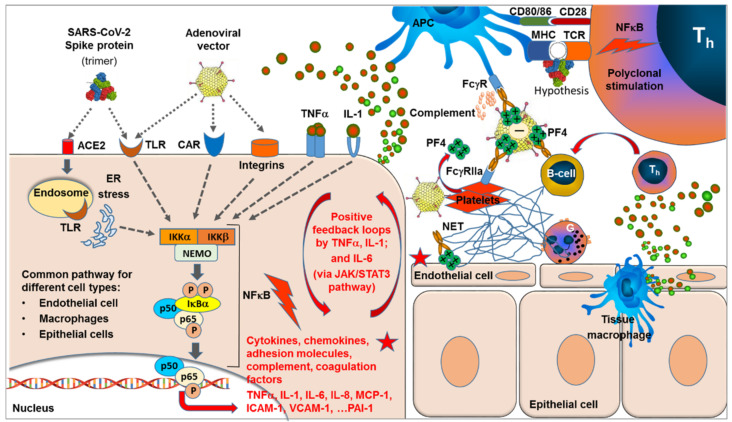
Figure 1.
SARS-CoV-2 spike protein (expressed following vaccination with either mRNA or adenoviral vector) binds to the ACE2 receptor followed by endocytosis. Binding of the adenovirus (including replication defective E1/E3 deletion variants) occurs via binding of the fiber-knob to the CAR (coxsackie and adenovirus receptor), followed by interaction between the arginine-glycine-aspartate (RGD) motif within the viral penton base with the integrins on the cell surface. In addition both, SARS-CoV-2 and adenovirus bind and activate various Toll-like receptors (TLRs), leading to activation of the NFκB signaling pathway, including activation of IKK complexes, release of p50/p65 complexes from the inhibitor complex with IκBa, and translocation of the phosphorylated p50/p65 heterocomplex to the nucleus, where the transcription of proinflammatory genes, including those for cytokines, such as TNFα, IL-1, and IL-6; chemokines, such as MCP-1 and MCP-3; adhesion molecules, such as ICAM-1 and VCAM-1; and complement components and coagulation factors, such as PAI-1, is induced. These processes are triggered in a broad variety of cell types that express the respective receptors, including epithelial cells, macrophages, and endothelial cells. Expression of TNFα and IL-1 and increased integrin expression can result in auto amplifying positive feedback loops, which may extend to other integrated systems such as complement and coagulation. Signaling of IL-6 via JAK/STAT3 pathway is expected to trigger additional amplifying loops. The release of chemokines, such as MCP-1 and IL-8, will attract the migration and accumulation of inflammatory and immune cells to the site. In particular, neutrophils are attracted, releasing DNA chromatin nets, i.e., NETs. While these events are known to accelerate and amplify in the case of massive viral infection by SARS-CoV-2, the localized expression of the spike protein in the case of vaccination was not expected to result in levels inducing a major systemic symptomatic picture. However, in the case of adenoviral vector vaccines, additive and synergistic effects are possible. In addition to additional activation of the NFκB pathway, adenoviruses directly bind and activate platelets, which causes their activation/aggregation. Activation of platelets results in increased release of PF4—a tetramer with high positive surface charge—which can bind to the negatively charged glycosaminoglycan on endothelial cells. VITT-induced antibodies are induced by electrostatic binding of the positively charged PF4 tetramers to the negative surface of the adenoviral vector hexones forming highly immunogenic complexes, which trigger the formation of anti-PF4-directed antibodies. Binding of induced antibodies to the PF4-attached adenoviral surface facilitates uptake of these complexes via an Fc-gammaR-mediated process. Antigen presenting cells (APC) take up antibody-PF4 (adenovirus) complexes via different types of Fc-gamma receptors, resulting in cellular activation and complement activation. Additionally, platelets are able to bind such complexes via their FcgammaR2a receptor, resulting in further activation, release of PF4, aggregation of platelets, and thromboses. The supposed superantigen features of the spike protein may present an additional mechanism for accelerating the induction of anti-PF4 antibodies. Different from antigen-specific activation of single T-cell clones in the case of conventional antigens, with the antigen presented in the MHC-TCR groove, superantigens bind outside this groove to the MHC and/or TCR, resulting in polyclonal activation. Adapted from ref. [38].