FIG 3.
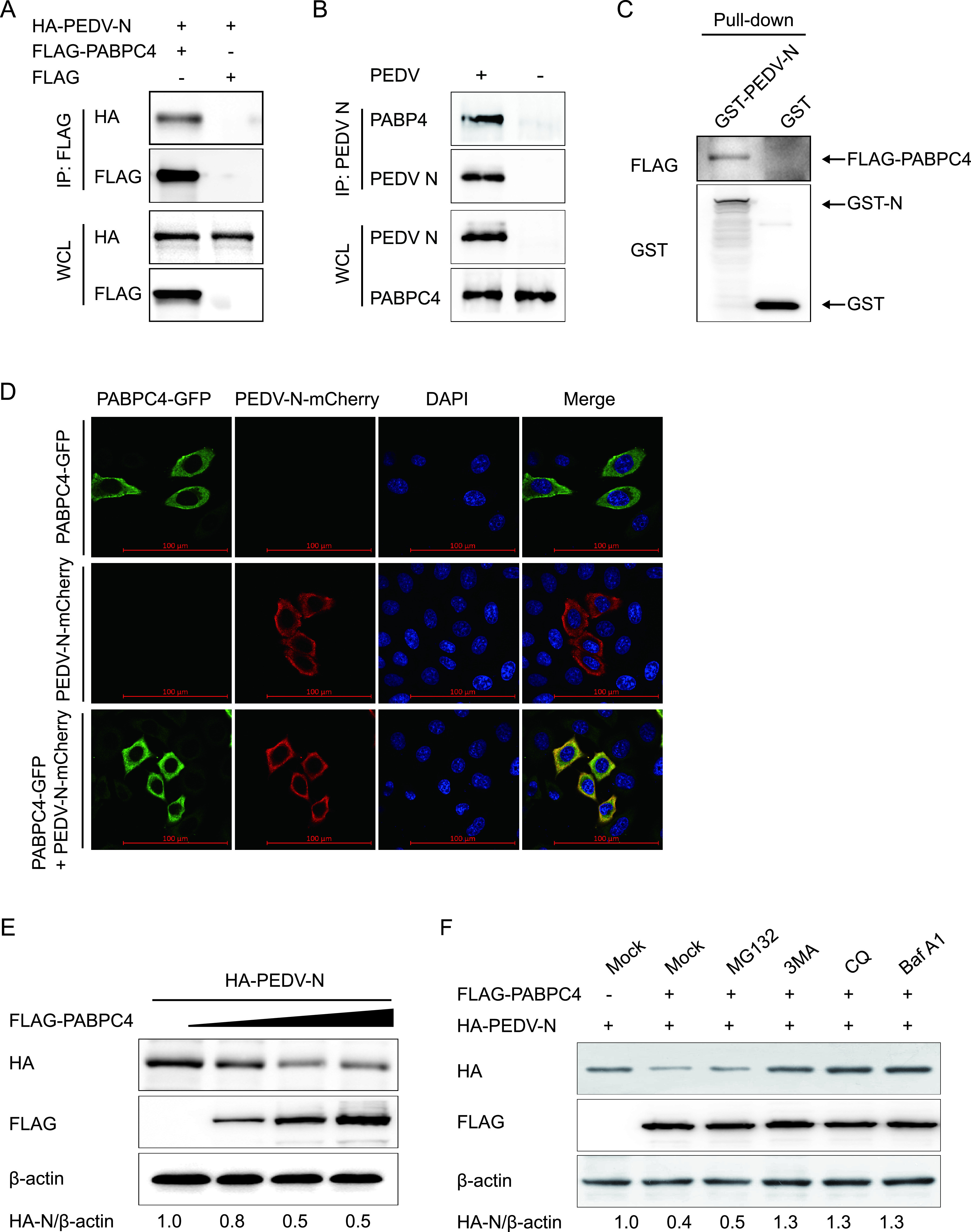
PABPC4 targets and degrades N protein by autophagy. (A) 293T cells were transfected with plasmids encoding FLAG-PABPC4 and HA-N for 24 h, and samples were then harvested and analyzed by co-IP with anti-FLAG binding beads and Western blotting. (B) Vero cells were infected or mock-infected with PEDV at an MOI of 1 and harvested at 24 hours postinfection (hpi). Immunoprecipitation was performed with an anti-PEDV N protein antibody, and Western blotting analysis was performed with a monoclonal antibody against PEDV N protein and an anti-PABPC4 antibody. (C) The PABPC4 protein and N protein were expressed in bacterial strain BL21(DE3) and purified for the GST pulldown analysis. Input, PABPC4. (D) HeLa cells were transfected with plasmids encoding PABPC4-GFP and N-mCherry for 24 h. The cells were fixed and processed for immunofluorescence. Fluorescent signals were observed with confocal immunofluorescence microscopy. Scale bars, 100 μm. (E) The increasing concentrations of a vector expressing FLAG-PABPC4 (wedge) and the vector expressing HA-N were transfected into 293T cells. The cell lysates were analyzed with Western blotting 24 h later. β-actin was used as the sample loading control. (F) Plasmids encoding FLAG-PABPC4 and HA-N were transfected into 293T cells and then treated with MG132 (5 μM), 3MA (0.5 mM), CQ (10 μM), or Baf A1 (0.1 μM) for 8 h. The cell lysates were then analyzed by Western blotting.
