FIG 4.
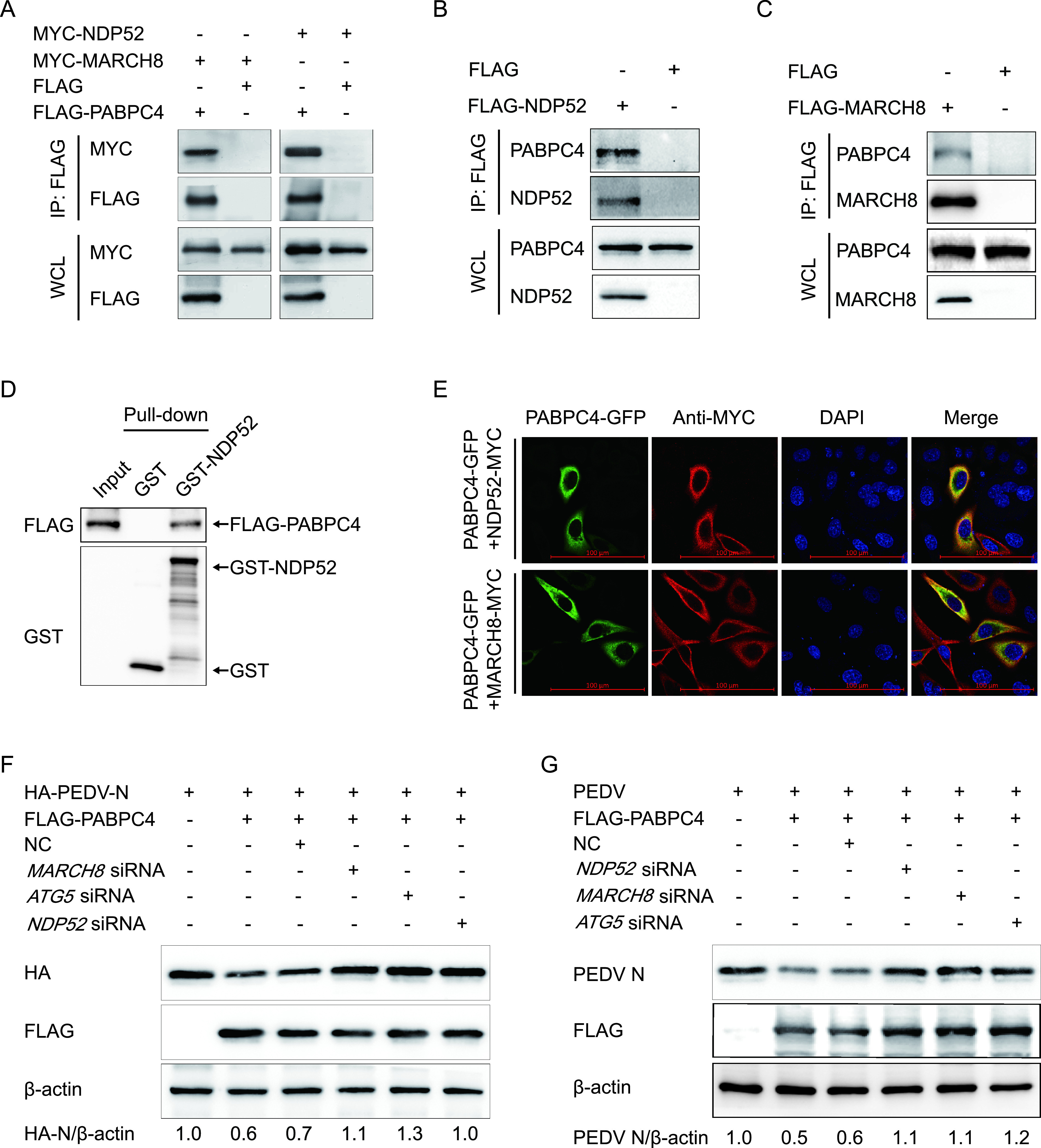
PABPC4 degrades PEDV N protein through selective autophagy. (A) 293T cells were transfected with plasmids encoding FLAG-PABPC4 and MYC-MARCH8 or MYC-NDP52 for 24 h, followed by co-IP with anti-FLAG binding beads and Western blotting with anti-MYC and anti-FLAG antibodies. The whole-cell lysates (WCLs) without immunoprecipitation were analyzed by Western blotting, and β-actin was used as the loading control. (B and C) LLC-PK1 cells were transfected with plasmids encoding FLAG-NDP52 or FLAG-MARCH8 for 24 h, followed by co-IP with anti-FLAG binding beads and Western blotting with anti-PABPC4, anti-NDP52, and anti-MARCH8 antibodies. (D) FLAG-PABPC4 and NDP52 genes were cloned into the pCold TF plasmid and pCold GST plasmid, respectively. Recombinant proteins were expressed in bacterial strain BL21(DE3) and purified for the GST pull-down. After adequate washing, proteins eluted from beads were analyzed by Western blotting. Input, FLAG-PABPC4. (E) 293T cells were transfected with plasmids encoding PABPC4-GFP and MYC-MARCH8 or MYC-NDP52 for 24 h and then probed with specific primary and secondary antibodies. Nuclei were stained with DAPI (blue). The fluorescent signals were observed with confocal immunofluorescence microscopy. Scale bars represent 100 μm. (F) 293T cells were cotransfected with small interfering RNA (NDP52 siRNA, MARCH8 siRNA, ATG5 siRNA, or negative-control siRNA) and plasmids encoding FLAG-PABPC4 and HA-PEDV-N and were then analyzed by Western blotting with anti-HA antibody. (G) Vero cells were cotransfected with plasmids encoding FLAG-PABPC4 and small interfering RNA (NDP52 siRNA, MARCH8 siRNA, ATG5 siRNA, or negative-control siRNA) and then infected with PEDV (MOI = 0.01) and harvested at the indicated times. Western blotting was performed with a monoclonal antibody against PEDV N protein.
