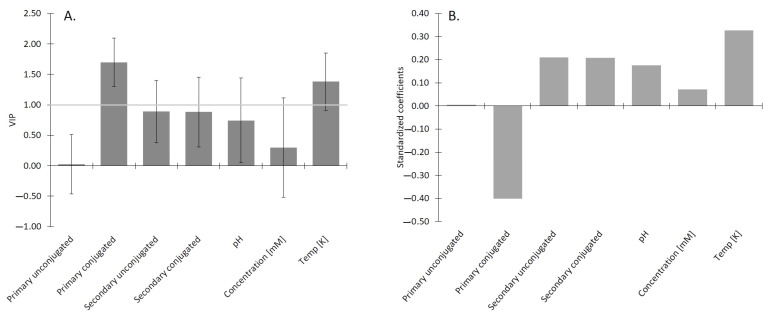
Figure 5.
(A) The most influential factors influencing the aggregation number are types of the BS, and the temperature. CMC of the BS tends to decrease with increasing temperature up to 303 K, beyond which the CMC starts to increase, leading to an increasing aggregation number [47]. (B) Conjugated forms of the BS have a tendency to have lower CMC than their unconjugated forms, therefore, the aggregate number for the conjugated BS should be smaller than for unconjugated ones. The primary conjugated BS showed a high negative correlation towards aggregation number. Conjugated forms of the BS are stabilized not only by the hydrophobic interaction but also by the hydrogen bonding, which means that they require fewer molecules than their unconjugated forms. The positive relation of the concentration [mM] of BS towards aggregation number yields the relation that with increasing BS concentration, the number of the incorporated molecules will increase [42].