FIG 1.
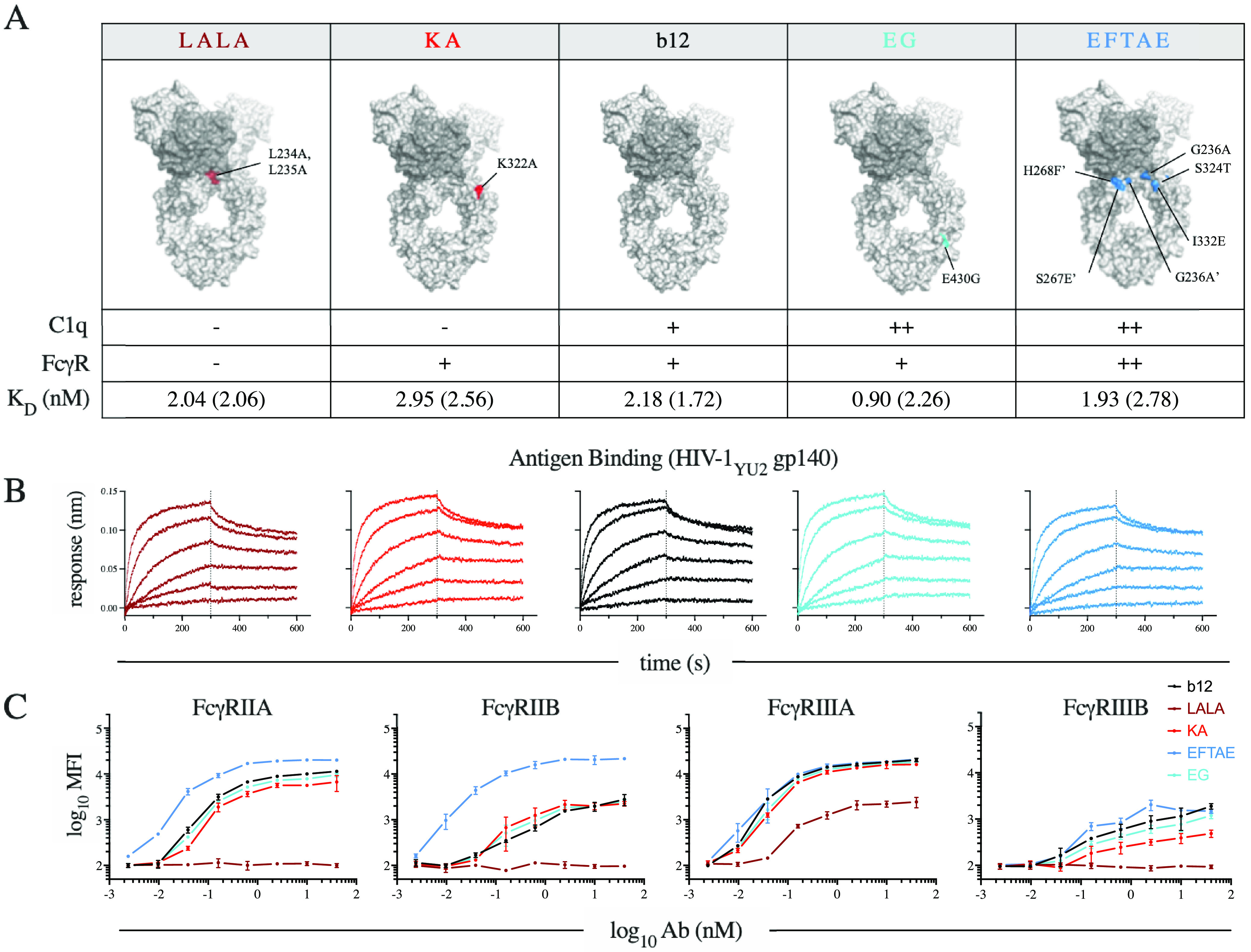
b12 variant panel. (A) Antibody (Ab) IgG1 Fc variants illustrated on the crystal structure of the broadly neutralizing antibody b12 (PDB accession number 1HZH) by coloration of the component point mutations. The phenotypically diminished variants to the left of b12 are illustrated in red (e.g., LALA and KA), while phenotypically enhanced variants to the right are in blue (e.g., EG and EFTAE). Accompanying the illustrations is a table of expected qualitative C1q and FcγR binding phenotypes and observed affinity of antigen binding (equilibrium [kinetic] dissociation constant [KD] values) of each variant to antigen (HIV-1YU-2 gp140 trimer). (B) Antigen binding profiles determined by biolayer interferometry (BLI) across a range of concentrations. (C) FcγR binding profiles of each variant as determined by staining antigen-conjugated beads with tetramerized receptor. Error bars represent the ranges from two technical replicates.
