Abstract
Abstract
Plants synthesize a vast array of specialized metabolites that primarily contribute to their defense and survival under adverse conditions. Many of the specialized metabolites have therapeutic values as drugs. Biosynthesis of specialized metabolites is affected by environmental factors including light, temperature, drought, salinity, and nutrients, as well as pathogens and insects. These environmental factors trigger a myriad of changes in gene expression at the transcriptional and posttranscriptional levels. The dynamic changes in gene expression are mediated by several regulatory proteins that perceive and transduce the signals, leading to up- or down-regulation of the metabolic pathways. Exploring the environmental effects and related signal cascades is a strategy in metabolic engineering to produce valuable specialized metabolites. However, mechanistic studies on environmental factors affecting specialized metabolism are limited. The medicinal plant Catharanthus roseus (Madagascar periwinkle) is an important source of bioactive terpenoid indole alkaloids (TIAs), including the anticancer therapeutics vinblastine and vincristine. The emerging picture shows that various environmental factors significantly alter TIA accumulation by affecting the expression of regulatory and enzyme-encoding genes in the pathway. Compared to our understanding of the TIA pathway in response to the phytohormone jasmonate, the impacts of environmental factors on TIA biosynthesis are insufficiently studied and discussed. This review thus focuses on these aspects and discusses possible strategies for metabolic engineering of TIA biosynthesis.
Purpose of work
Catharanthus roseus is a rich source of bioactive terpenoid indole alkaloids (TIAs). The objective of this work is to present a comprehensive account of the influence of various biotic and abiotic factors on TIA biosynthesis and to discuss possible strategies to enhance TIA production through metabolic engineering.
Keywords: Terpenoid indole alkaloids, Catharanthus roseus, Specialized metabolites, Biotic and abiotic factors, Gene regulation, Metabolic engineering
Introduction
The medicinal plant Catharanthus roseus is the source of almost 200 terpenoid indole alkaloids (TIAs), including the anticancer therapeutics vinblastine and vincristine (De Luca et al. 2014). The pharmaceutically important TIAs, vinblastine and vincristine, accumulate in extremely low quantities in C. roseus, leading to research efforts to enhance production through various strategies. Towards this end, the TIA biosynthetic pathway has been extensively studied, and the genes encoding key enzymes in the pathway have been identified and characterized (Fig. 1) (Miettinen et al. 2014; Qu et al. 2015, 2018, 2019; Stavrinides et al. 2016). The regulation of the TIA biosynthetic pathway is highly complex and the subject of current research (Patra et al. 2013; Thamm et al. 2016). Biosynthetic genes and transcriptional regulators, either individually or in combination, have been used to engineer the TIA pathway (Sharma et al. 2020; Schweizer et al. 2018; Tang and Pan 2017; Zhao and Verpoorte 2007; Zárate and Verpoorte 2007; Hughes et al. 2004; Hughes and Shanks 2002; Morgan and Shanks 2000; Rijhwani and Shanks 1998; Peebles et al. 2009). As a protocol for regeneration of transgenic C. roseus plants is not well established, cell lines and hairy roots are extensively used in the majority of these studies. Recently, transient transformation of C. roseus seedlings and flower petals have also been explored (Liu et al. 2019; Schweizer et al. 2018; Singh et al. 2020, 2021). There are only a few reports on the characterization of TIA pathway genes using transgenic plants (Pan et al. 2012; Sharma et al. 2018b). In general, two bioengineering strategies are used to boost TIA production in C. roseus (Sharma et al. 2020). One approach is to “push” the metabolic flux towards downstream by increasing the precursor pool through overexpressing genes encoding the upstream or midstream rate-limiting enzymes and associated TFs. The other is to “pull” the metabolic flux towards the final products through manipulating the downstream biosynthetic genes. A more effective approach is perhaps to simultaneously “push-and-pull” by upregulating both upstream and downstream genes. A limitation to such an approach is the requirement of transforming a large number of genes, currently a significant engineering challenge. Increasing evidence shows that certain environmental signals tend to trigger the upstream, midstream, and downstream TIA biosynthetic genes and regulators. Here, we discuss whether the knowledge regarding the impacts of environmental factors on TIA pathway can be explored for metabolic engineering to increase TIA production.
Fig. 1.
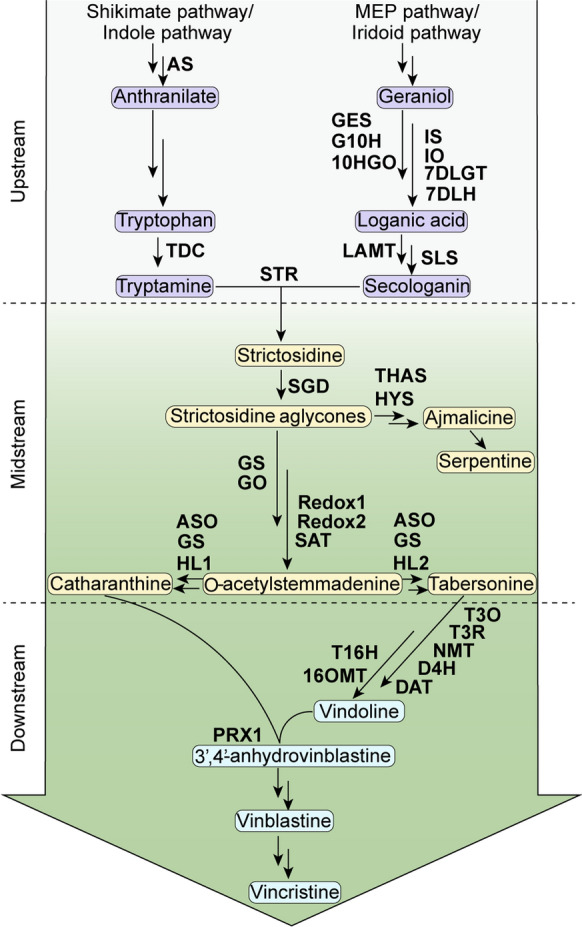
Schematic diagram of the TIA biosynthetic pathway in C. roseus, which is divided into three stages: upstream, midstream, and downstream as shown. ASα anthranilate synthase, ASO O-acetylstemmadenine oxidase, D4H desacetoxyvindoline-4-hydroxylase, DAT deacetylvindoline-4-O-acetyltransferase, 7DLGT 7-deoxyloganetic acid glucosyl transferase, 7DLH 7-deoxyloganic acid hydroxylase, G10H geraniol 10-hydroxylase, GES geraniol synthase, GO geissoschizine oxidase, GS geissoschizine synthase, HL1/2 hydrolase 1/2, HYS heteroyohimbine synthase, 10HGO 10-hydroxygeraniol oxidoreductase, IO iridoid oxidase, IS iridoid synthase, LAMT loganic acid methyltransferase, NMT 3-hydroxy-16-methoxy-2,3-dihydrotabersonine-N-methyltransferase, 16OMT 16-hydroxytabersonine-O-methyltransferase, PRX1 peroxidase 1, SAT stemmadenine-O-acetyltransferase, SGD strictosidine β-glucosidase, SLS secologanin synthase, SS serpentine synthase, STR strictosidine synthase, T3O tabersonine 3-oxygenase, T3R tabersonine 3-reductase, T16H2 tabersonine 16-hydroxylase 2, TDC tryptophan decarboxylase, THAS tetrahydroalstonine synthase
TIA biosynthetic pathway and the complex gene regulation
The TIA pathway can be broadly divided into three parts: the upstream, midstream, and downstream (Fig. 1). The products of the upstream and midstream pathways, such as strictosidine, ajmalicine, serpentine, catharanthine, and tabersonine, are accumulated in various tissues in the whole plant (van Der Heijden et al. 2004). However, products of the downstream pathway, including vindoline, anhydrovinblastine, vinblastine, and vincristine, are mainly accumulated in the aerial tissues (DeLuca et al. 1986). Two distinct branch pathways provide the precursors for TIA biosynthesis: the shikimate pathway supplies the indole moiety tryptamine, and the methylerythritol pathway (MEP)/iridoid pathway generates the terpenoid moiety secologanin. TIA biosynthesis is highly compartmentalized, occurring in at least four cell types and different subcellular compartments (Courdavault et al. 2014). Biosynthesis of secologanin overlaps between internal phloem associated parenchyma (IPAP) and epidermal cells. Three nitrate/peptide family (NPF) transporters, CrNPF2.4, CrNPF2.5 and CrNPF2.6, are involved in the intracellular transport of multiple iridoid intermediates (Larsen et al. 2017). Biosynthesis of tryptamine occurs in cytosol of the epidermal cells. Secologanin and tryptamine are coupled to form strictosidine in the vacuoles, and then exported to cytosol through the tonoplast-localized NPF transporter CrNPF2.9 (Payne et al. 2017). Strictosidine is then deglucosylated by the nuclear-localized glucosidase, strictosidine ß-D-glucosidase (SGD), to form the strictosidine aglycone, which is converted to reactive dialdehyde that serves as a precursor for the biosynthesis of complex TIAs, including ajmalicine, serpentine, catharanthine, and tabersonine (Guirimand et al. 2010). Catharanthine is secreted out to leaf surface by the ABC transporter CrTPT2 (Yu and De Luca 2013). Tabersonine is further converted to vindoline through a seven-step enzymatic process, occurring in laticifers and idioblasts in the leaf (Qu et al. 2015). Vinblastine and vincristine are derived from the coupling of catharanthine and vindoline. In roots, tabersonine is converted to hörhammericine, catalyzed by tabersonine 6,7-epoxidase isoforms 1 and 2 (TEX1/2), tabersonine 19-hydroxylase (T19H), and tabersonine derivative 19-O-acetyltransferase (TAT) (Carqueijeiro et al. 2018a, b; Giddings et al. 2011).
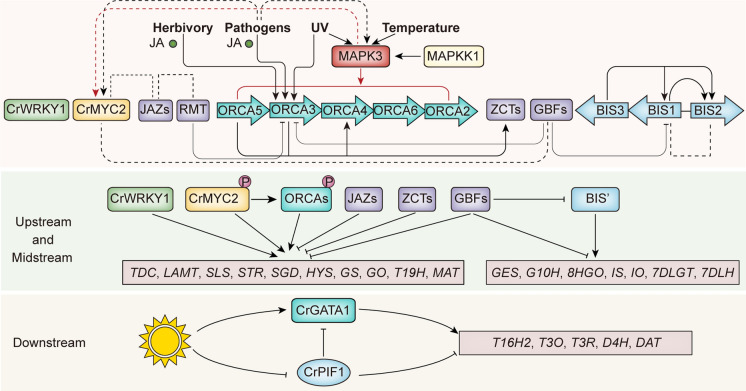
The phytohormone jasmonate (JA) and its methyl esters MeJA are key elicitors of TIA biosynthesis. The key components of JA signaling, including the JA co-receptor CORONATINE INSENSITIVE 1 (COI1) and the five JASMONATE ZIM-domain proteins CrJAZ1/2/3/8/10, have been characterized for their roles in regulating TIA biosynthesis (Patra et al. 2018). A number of JA-responsive transcription factors (TFs) have been identified as regulators of the TIA pathway (Fig. 2). These TFs include transcription activators from the TF families of bHLH (CrMYC2, BIS1/2/3) (Zhang et al. 2011; Van Moerkercke et al. 2015, 2016; Singh et al. 2021), AP2/ERF (ORCA2/3/4/5/6, CrERF5) (Singh et al. 2020; Paul et al. 2017, 2020; Pan et al. 2019; van der Fits and Memelink 2000; Li et al. 2013; Menke et al. 1999), and WRKY (CrWRKY1) (Suttipanta et al. 2011), as well as transcription repressors from the TF families of bZIP (GBF1/2) (Sibéril et al. 2001; Sui et al. 2018), zinc finger factors (ZCT1/2/3) (Pauw et al. 2004), bHLH (RMT1) (Patra et al. 2018), and AP2/ERF (CR1) (Liu et al. 2017a). The repressors, ZCTs, RMT1 and GBF1/2, are induced by the transcriptional activators ORCAs, BIS1 and/or CrMYC2 (Sui et al. 2018; Patra et al. 2018; Van Moerkercke et al. 2015; Paul et al. 2017; Peebles et al. 2009). In addition to JA, other phytohormones and environmental factors regulate TIA biosynthesis. Two light-responsive TFs, CrGATA1 and CrPIF1, act as a transcriptional activator and repressor, respectively, to regulate vindoline biosynthesis (Liu et al. 2019) (Fig. 2). However, compared to our understanding of the TIA pathway regulation in response to JA, mechanistic studies on biotic and abiotic factors affecting TIA metabolism are limited. Here, we discuss our current understanding of the effects of environmental factors on TIA biosynthesis.
Fig. 2.
Transcriptional and posttranscriptional regulation of the TIA pathway and influence of environmental factors on TIA biosynthesis. Top panel: complex regulation of the TIA pathways. Environmental factors, such as herbivores, pathogens, UV, and temperature influence TIA biosynthesis by modulating the expression of TFs (e.g., ORCA3 and MYC2) and pathway genes. ORCAs (ORCA2, ORCA, ORCA4, ORCA5, and ORA6) and BIS’ (BIS1, BIS2, and BIS3) are present as clusters in C. roseus genome and exhibit intra-cluster regulation (e.g., ORCA5 activates ORCA3 and 4). The ORCAs, CrMYC2, BIS’, CrWRKY1, and CrGATA1 are transcriptional activators, whereas GBFs, ZCTs, JAZs, RMT and CrPIF1 are repressors of the TIA pathway. Middle panel: selective and post-translational regulations of upstream and midstream pathway genes. CrMYC2, ORCAs, and CrWRKY1 regulate upstream indole branch and the midstream pathway genes (from TDC to MAT), whereas BIS’ regulate the iridoid branch genes (from GES to 7DLH). Many TFs are phosphorylated, leading to functional alteration. The MAPKK1-MPK3 cascade phosphorylate CrMYC2 and ORCAs to regulate their activity. MPK3 expression and kinase activity are induced by pathogens (including fungal endophytes) and temperature. GBFs repress the activities of ORCA3 and BIS1. RMT1 represses the ORCA3 activity on target gene promoters. Lower panel: light regulation of the vindoline pathway. CrGATA1 and CrPIF1 regulate the downstream vindoline pathway. Light (in particular, red light) activates CrGATA1, while causes the degradation of CrPIF1, a repressor of CrGATA1. Protein-protein interaction between CrMYC2-GBFs, CrMYC2-JAZs, RMT-JAZ, and BIS1-BIS2 are indicated by dotted lines. Solid blue arrows indicate direct activation, dotted arrows indicate indirect or unclarified activation, and T-bars represent repression. Phosphorylation of ORCAs and MYC2 is indicated by circled P. BIS1/2/3 bHLH iridoid synthesis 1/2/3, MAPKK1 mitogen-activated protein kinase kinase 1, MPK3 mitogen-activated protein kinase 3, GBFs G-box binding factors, JAZ jasmonate ZIM domain proteins, ORCA2/3/4/5/6 octadecanoid-derivative responsive Catharanthus AP2-domain, ZCT zinc finger Catharanthus transcription factors, CrPIF1 Catharanthus roseus phytochrome-interacting factor 1, RMT1 repressor of MYC2 targets 1, UV ultraviolet
Regulation of TIA biosynthesis by environmental factors
Light
Light regulates plant development and the biosynthesis of many specialized metabolites, such as anthocyanins and artemisinin, mediated by several TFs (Liu et al. 2015; Hao et al. 2019; Li et al. 2016). MAP Kinase 4 (MPK4) and a R2R3 MYB TF, Production of Anthocyanin Pigment 1 (PAP1), regulate light-induced accumulation of anthocyanin in Arabidopsis (Li et al. 2016). Light-induced accumulation of artemisinin in Artemisia annua is regulated by the bZIP TF HY5 (Hao et al. 2019). In C. roseus, vindoline biosynthesis is regulated by light (Liu et al. 2019; DeLuca et al. 1986). Dark-grown, etiolated C. roseus seedlings accumulate a trace amount of vindoline, which increases upon exposure to light (DeLuca et al. 1986). The accumulation of TIAs is correlated with the increase in gene expression and enzyme activities of desacetoxyvindoline-4-hydroxylase (D4H) and deacetylvindoline-4-O-acetyltransferase (DAT) upon exposure to light in C. roseus seedlings (Table 1) (St-Pierre et al. 1998; De Carolis et al. 1990). The GATA family TF, CrGATA1, is an activator, while CrPIF1 is a negative regulator, of vindoline biosynthesis. Upon exposure of C. roseus seedlings to light, CrGATA1 upregulates tabersonine 16-hydroxylase 2 (T16H2), tabersonine 3-oxygenase (T3O), tabersonine 3-reductase (T3R), D4H, and DAT. CrPIF1 represses the expression of T16H2 and DAT in dark. Moreover, CrPIF1 represses the expression of CrGATA1. Derepression of CrGATA1, presumably by light-induced degradation of CrPIF1, enhances the expression of five vindoline pathway genes, leading to increased vindoline accumulation (Liu et al. 2019).
Table 1.
The effects of environmental factors on TIA biosynthesis in C. roseus
| Environmental factors | Plant materials | Regulated genes | Metabolites | References |
|---|---|---|---|---|
| Light | Seedlings | DAT (↑)* | Vindoline (↑)* | DeLuca et al. (1986), St-Pierre et al. (1998) |
| Seedlings | D4H (↑) | – | De Carolis et al. (1990) | |
| Seedlings | CrPIF1 (↑), CrGATA1 (↑), T16H2 (↑), T3O (↑), T3R (↑), D4H (↑), DAT (↑) | Vindoline (↑) | Liu et al. (2019) | |
| Drought | Shoots | – | Vincristine (↑) | Osman et al. (2007) |
| Roots | – | Ajmalicine (↑) | Jaleel et al. (2008b) | |
| Seedlings | – | Total TIAs (↑)Vinblastine (↑)Vincristine (↑) | Amirjani (2013) | |
| Leaves | STR (↑) | – | Dutta et al. (2013) | |
| Seedlings | TDC (↑), STR (↑), DAT (↑) | Catharanthine (↑)Vindoline (↑)Vinblastine (↑) | Liu et al. (2017b) | |
| Leaves | – | Vinblastine (↑)Vincristine (↑) | Ababaf et al. (2021) | |
| Salt | Shoots | – | Vincristine (↑) | Osman et al. (2007) |
| Roots | – | Ajmalicine (↑) | Jaleel et al. (2008c) | |
| Roots | – | Ajmalicine (↑) | Jaleel et al. (2008a) | |
| Leaves | – | Total TIAs (↓)Vinblastine (↓)Vincristine (↓) | Idrees et al. (2011) | |
| Leaves | STR (↑) | Catharanthine (↓)Vindoline (↓)Vinblastine (↓)Vincristine (↑) | Dutta et al. (2013) | |
| Leaves | D4H (↑), DAT (↑) | – | Mokhaberi et al. (2013) | |
| Cultivated tissues | – | Vinblastine (↑)Vincristine (↑) | Fatima et al. (2015) | |
| Hight temperature | Leaves | – | Catharanthine (↑)Vindoline (↑)Vinblastine (↑) | Guo et al. (2007) |
| Leaves | CrMPK3 (↑) | – | Raina et al. (2013) | |
| Low temperature | Leaves | TDC (↓), D4H (↓) | Catharanthine (↓)Vindoline (↓)Vinblastine (↓) | Dutta et al. (2007) |
| Leaves | STR (↓) | Catharanthine (↓)Vindoline (↓)Vinblastine (↓)Vincristine (↓) | Dutta et al. (2013) | |
| Ultraviolet | Leaves | TDC (↑), STR (↑) | Total TIAs (↑) | Ouwerkerk et al. (1999a, b) |
| Suspension cells | TDC (↑), STR (↑) | Catharanthine (↑) | Ramani and Chelliah 2007) | |
| Suspension cells | – | Catharanthine (↑)Vindoline (↑) | Ramani and Jayabaskaran 2008) | |
| Hairy roots | G10H (↑) | Total TIAs (↑) | Binder et al. (2009) | |
| Seedlings | – | Catharanthine (↑)Vindoline (↑)Vinblastine (↑) | Guo et al. (2014) | |
| Leaves | G10H (↑), TDC (↑), STR (↑), ORCA3 (↑), T16H (↑), D4H (↑), DAT (↑) | Strictosidine (↑)Ajmalicine (↑)Catharanthine (↑)Vindoline (↑) | Zhu et al. (2015) | |
| Cultivated plantlets | – | Vincristine (↑) | Salama et al. (2020) | |
| Leaves | – | Ajmalicine (↑)Vinblastine (↑)Vincristine (↑) | Zhong et al. (2021) | |
|
Heavy metal Vanadium |
Suspension cells | – | Ajmalicine (↑)Catharanthine (↑) | Smith et al. (1987) |
| Cadmium | Leaves | – | Catharanthine (↑)Vindoline (↑)Vinblastine (↑) | Chen et al. (2018) |
| Leaves and roots | – | Ajmalicine (↓)Vindoline (↓) | Srivastava and Srivastava (2010) | |
| Suspension cells | TDC (↑) | Ajmalicine (↑) | Zheng and Wu (2004) | |
| Nickel, manganese | Roots and leaves | – | Ajmalicine (↓)Vindoline (↓) | Srivastava and Srivastava (2010) |
| Lead | Leaves | – | Vindoline (↓) | Srivastava and Srivastava (2010) |
| Chromium | Shoots | – | Vinblastine (↓)Vincristine (↑) | Rai et al. (2014) |
| Cobalt | Suspension cells | – | Total TIAs (↑) | Fouad et al. (2018) |
|
Nutrient deficiency Nitrogen, phosphorus, magnesium, sulfur |
Roots | – | Ajmalicine (↓) | Mendonça Freitas et al. (2016) |
| Potassium | Roots | – | Ajmalicine (↑) | |
| Herbivore(Manduca sexta) | Leaves | ORCA3 (↑), STR (↑), SGD (↑), D4H (↑), DAT (↑) | Total TIAs (↑)Ajmalicine (↑)Catharanthine (↑)Vindoline (↑) | De Bernonville et al. (2017) |
|
Pathogens Aspergillus niger, Fusarium moniliforme, Trichoderma viride |
Suspension cells | – | Ajmalicine (↑) | Namdeo et al. (2002) |
| Fusarium oxysporum | Suspension cells | TDC (↑) | Total TIAs (↑) | Tang et al. (2011) |
| Aspergillus flavus | Callus tissues | – | Vinblastine (↑)Vincristine (↑) | Tonk et al. (2016) |
| Yeast extract | Callus tissues | – | Vinblastine (↑)Vincristine (↑) | Maqsood and Abdul 2017) |
| Pseudomonas fluorescens, Azospirillum brasilense | Roots | TDC (↑), STR (↑) | – | Ahmadzadeh et al. (2020) |
| Curvularia sp. CATDLF5, Choanephora infundibulifera CATDLF6 | Leaves | G10H (↑), TDC (↑), STR (↑), 16OMT (↑), D4H (↑), DAT (↑), PRX1 (↑), ORCA3 (↑), ZCTs (↓) | Vindoline (↑) | Pandey et al. (2016) |
Up and down arrows indicate increase and decrease of gene expression and metabolite accumulation, respectively
Drought and Salinity
Drought and salt stresses affect plant growth, morphology and metabolic processes. Adaptations to drought and salt stresses involve changes in metabolic processes, including biosynthesis and accumulation of primary and specialized metabolites, that promote drought and salt resistance (Zahedi et al. 2019). In Arabidopsis, drought induces the accumulation of glucosinolates, while salt stress increases the accumulation of flavonoids (Salehin et al. 2019; Li et al. 2019a). In C. roseus, drought or salt stress increases the accumulation of TIAs, including ajmalicine, catharanthine (Liu et al. 2017b; Jaleel et al. 2008a, b, c), vindoline, vinblastine, and vincristine (Liu et al. 2017b; Amirjani 2013; Osman et al. 2007; Fatima et al. 2015; Dutta et al. 2013; Ababaf et al. 2021) (Table 1). Consistent with the increase of TIAs, expression of both upstream (TDC and STR) and downstream (D4H and DAT) TIA pathway genes are induced by drought or salt stress. However, it is unclear how these pathway genes are regulated by stress signal transduction and gene transcription.
Phytohormones play important roles in abiotic stress response in plants (Ullah et al. 2018). Abscisic acid (ABA) is the key phytohormone which intensifies drought and salt tolerance in plants. The SnRK2 protein kinases and protein phosphatases 2 C (PP2C) are important components of the ABA signaling pathway. Under normal conditions (low ABA content), PP2Cs interact with and dephosphorylate SnRK2s to inhibit ABA response. When the ABA level increases in response to drought or salt stress, PP2C dissociate from SnRK2 which is auto-phosphorylated and then phosphorylate the downstream targets to promote ABA responses (Ullah et al. 2018). In response to ABA, a SnRK2 kinase from A. annua (AaAPK1) phosphorylates a bZIP TF, AabZIP1, to activate artemisinin biosynthesis, while a PP2C-type phosphatase, AaPP2C1, negatively regulates artemisinin biosynthesis through dephosphorylation of AaAPK1 (Zhang et al. 2018, 2019). ABA also promotes catharanthine production in C. roseus suspension cells (Chen et al. 2013). It is possible that drought or salt stress triggers ABA signaling that activates SnRK2s to promote TIA accumulation.
Temperature
Both low and high temperature limit plant growth and development by reprograming various metabolic processes. Temperature affects the accumulation of specialized metabolites, such as flavonoids and phenolic compounds, which possibly play roles in temperature tolerance (Cohen and Kennedy 2010; Chalker-Scott 1999). In Arabidopsis, anthocyanin accumulation is induced by low temperature and suppressed by high temperature (Kim et al. 2017). Artemisinin biosynthesis in A. annua is also induced by cold and regulated by a TF module comprising the TFs, AabHLH112 and AaERF1 (Xiang et al. 2019). In C. roseus leaves, accumulation of midstream and downstream metabolites, including catharanthine, vindoline and vinblastine, is increased by high temperature (Guo et al. 2007) and suppressed by low temperature (Dutta et al. 2007, 2013) (Table 1). Consistent with the cold-induced suppression of metabolites, expression of STR, TDC, and D4H is also decreased (Dutta et al. 2007, 2013). Interestingly, a heat-activated MAPK, CrMAPK3 (Raina et al. 2013), induces the expression both upstream (TDC and STR) and downstream (D4H and DAT) TIA biosynthetic genes in C. roseus leaves (Raina et al. 2012) (Fig. 2). We have reported that CrMAPK3 and CrMAPK6 likely phosphorylate CrMYC2 and ORCAs to induce TIA biosynthetic genes, such as TDC and STR (Paul et al. 2017). In Arabidopsis, MAPK3 and MAPK6 are important components in cold signaling pathway (Li et al. 2017). These findings suggest that severe temperature possibly regulate TIA biosynthesis through the CrMAPK3/6 signaling pathway.
Ultraviolet
Ultraviolet (UV) radiation (200–400 nm) can be classified into UV-A (320–400 nm), UV-B (280–320 nm), and UV-C (200–280 nm). Accumulation of specialized metabolites, such as phenolic compounds and flavonoids, serves as a common protective mechanism against potentially damaging UV irradiation to plants (Zhang and Björn 2009; Frohnmeyer and Staiger 2003). Exposure of Arabidopsis seedlings to UV-B (8.0 kJ m− 2 day− 1) for 6 h significantly induces the expression of key phenylpropanoid pathway genes, such as phenylalanine ammonia-lyase (PAL) and chalcone synthase (CHS). Longer exposure to UV increases the accumulation of flavonoids and sinapate compounds, suggesting their roles in UV protection (Li et al. 1993). Short-term (14 days) exposure to UV-B (4.2 kJ m− 2 day− 1) and UV-C (5.7 kJ m− 2 day− 1) also induces accumulation of flavonoids and artemisinin in leaves and inflorescences of A. annua (Rai et al. 2011). In the medicinal plant water mint (Mentha aquatica), prolonged UV-B exposure (2 or 4 h daily for 3 weeks) alters the volatile oil profile and increases the accumulation of phytochemicals (Nazari and Zarinkamar 2020). TIAs are known to absorb UV and function as UV protectants (Ouwerkerk et al. 1999b). UV-B induces the accumulation of ajmalicine, catharanthine, and vindoline in C. roseus suspension cells or hairy roots (Table 1) (Binder et al. 2009; Ramani and Jayabaskaran 2008; Ramani and Chelliah 2007). UV-A or UV-B irradiation also leads to increased accumulation of strictosidine, catharanthine, vindoline, vinblastine, and vincristine in C. roseus leaves or shoot cultures (Salama et al. 2020; Guo et al. 2014; Ouwerkerk et al. 1999b; Zhu et al. 2015). UV treatment induces the expression of pathways genes, including G10H, 10HGO, TDC, STR, T16H, D4H, and DAT, and the TF ORCA3 (Ouwerkerk et al. 1999a; Binder et al. 2009; Zhu et al. 2015) (Fig. 2). A recent study on the effects of UV-B on the mitochondria and plastid proteomes of C. roseus shows the increase of proteins related to the MEP pathway, that provides the monoterpene precursor. Additionally, consistent with the previous reports, UV-B exposure increases accumulation of ajmalicine, vincamine, deacetylvindoline, and vincristine in C. roseus leaves (Zhong et al. 2021). These findings collectively suggest that the UV-B receptor and the associated signal transduction pathway are involved in the regulation of the TIA pathway. Another study shows that expression and kinase activity of CrMAPK3 are induced by UV-C irradiation in C. roseus leaves (Raina et al. 2012), indicating that UV-induced TIA biosynthesis is possibly regulated by protein kinases (Fig. 2). Supporting this hypothesis, a recent study shows that UV-B exposure increases ATP content in C. roseus leaves and induces significant change in leaf phospho-proteome. Upon UV exposure, phosphoproteins related to protein synthesis/degradation/ modification, heat-shock proteins, and protein kinases, such as the calcium-dependent protein kinases, change significantly (Zhong et al. 2019).
Heavy metals
Studies show that heavy metals affect TIA accumulation in C. roseus. In suspension cells, vanadium (V), cadmium (Cd), and cobalt (Co) induce the production of ajmalicine and catharanthine (Table 1) (Zheng and Wu 2004; Smith et al. 1987; Fouad and Hafez 2018). Expression of TDC is induced by Cd in C. roseus suspension cells, which correlates to the increase of TIAs (Zheng and Wu 2004). Cd is also reported to induce the accumulation of catharanthine, vindoline and vinblastine in C. roseus leaves (Chen et al. 2018); however, this is in contrary to a previous study showing Cd, reduces vindoline contents in C. roseus leaves (Srivastava and Srivastava 2010). Chromium (Cr) treatment leads to an increase of vinblastine and vincristine in C. roseus leaves (Rai et al. 2014). Ni and Mn reduce vindoline content in leaves. Cadmium (Cd), Nickel (Ni) or manganese (Mn) treatment increases serpentine content by 2–3 fold, while suppresses ajmalicine in C. roseus roots (Srivastava and Srivastava 2010).
Nutrient deficiency
Nutrient deficiency affects not only plant growth and development, but also the biosynthesis of many specialized metabolites (Yang et al. 2018). For example, nutrient deficiency results in increased accumulation of anthocyanins in plants (Zhang et al. 2017; Wang et al. 2015; Ren et al. 2021). Nitrogen (N) is an essential nutrient for plant growth and development and a constituent of alkaloids. N fertilizers affect the accumulation of TIAs in C. roseus plants (Table 1) (Gholamhosseinpour et al. 2011). N deficiency reduces ajmalicine accumulation in C. roseus roots (Mendonça Freitas et al. 2016), while higher N supply reduces contents of catharanthine, vindoline and vinblastine in C. roseus leaves (Guo et al. 2014). In addition to N, deficiency of other nutrients also alters TIA production. Potassium (K) deficiency increases, while deficiencies of phosphorus (P), magnesium (Mg), and sulfur (S) decrease, ajmalicine accumulation in C. roseus roots (Mendonça Freitas et al. 2016).
Herbivores and pathogens
The plant specialized metabolites are defense molecules that confer resistance against pathogens and herbivores (Panda et al. 2021). Similarly, C. roseus produces TIAs in response to herbivore and pathogens as chemical defense (Fig. 2). TIAs, such as catharanthine and anhydrovinblastine, are toxic to herbivores and pathogens (De Bernonville et al. 2017; Roepke et al. 2010). The TIA pathway metabolites and corresponding genes are induced by the herbivory of Manduca sexta on C. roseus leaves (Table 1) (De Bernonville et al. 2017). In C. roseus suspension cells, the fungal pathogens, Aspergillus niger, Fusarium moniliforme, F. oxysporum and Trichoderma viride, induce TDC activity and the accumulation of total alkaloids (Tang et al. 2011; Namdeo et al. 2002). In C. roseus calli, yeast extract or A. flavus induces the accumulation of vinblastine and vincristine (Maqsood and Abdul 2017; Tonk et al. 2016). In C. roseus leaves, the fungal endophytes Curvularia sp. CATDLF5 and Choanephora infundibulifera CATDLF6 upregulate the expression of TIA pathway genes and the accumulation of vindoline (Pandey et al. 2016). In addition, expression of TDC and STR is induced in C. roseus roots after infection by two rhizobacteria, Pseudomonas fluorescens and Azospirillum brasilense (Ahmadzadeh et al. 2020).
The phytohormones JA, salicylic acid (SA), and ethylene are involved in plant disease resistance (Dong 1998). These phytohormones crosstalk with the MAPK cascades to confer disease resistance (Wang et al. 2013; Han et al. 2010; Zhang and Liu 2001). The homologous MAPK3 and 6 are emerging as key components in disease resistance by regulating various defense responses, including the induction of camalexin, a phytoalexin in Arabidopsis (Meng and Zhang 2013; Mao et al. 2011). We have also reported the critical roles of the CrMAPKK1-CrMAPK3/6 cascade in the regulation of TIA biosynthesis in C. roseus (Paul et al. 2017). Furthermore, in addition to the well characterized JA induction, the disease resistance associated hormones, SA and ethylene, also induce the production of TIAs in C. roseus leaves or seedlings (Soltani et al. 2020; Wang et al. 2016; Pan et al. 2010, 2015; Idrees et al. 2011; El-Sayed and Verpoorte 2004). Meanwhile, both upstream (G10H, TDC and STR) and downstream (T16H, D4H and DAT) genes are upregulated by SA and ethylene. Therefore, biotic factors possibly trigger TIA accumulation through the sophisticated crosstalk between phytohormone signaling and the CrMAPKK1-CrMAPK3/6 pathway.
Strategies for metabolic engineering TIA biosynthesis
The low-level accumulation of therapeutically important TIAs has intrigued researchers to develop innovative strategies to boost TIA production. Previous studies on the TIA pathway have identified the genes encoding enzymes and regulatory TFs. The genes encoding enzymes and TFs have been used for metabolic engineering of the TIA pathway with various degrees of success. Studies on the influences of environmental factors have provided limited but important information on changes in the expression profiles of TIA pathway genes and regulators. Here, we discuss whether the biotic or abiotic factor-responsive pathway genes and/or TFs can be used as tools to engineer TIA biosynthesis. In this section, we also describe several technology platforms and strategies used for TIA pathway engineering.
Resources
Both homologous and heterologous gene expression systems have been used to study the regulation and metabolic engineering of TIA pathway. As the generation of stable transgenic C. roseus plants is not well established, suspension cells and hairy roots serve as effective platforms for studying TIA biosynthesis and regulation. However, the cell lines or hairy roots only produce upstream and midstream metabolites due to the lack of precursors or extremely low expression of the upstream pathway genes, limiting their uses in engineering of downstream TIAs. For instance, some of the cell lines (e.g., MP183L) do not produce any alkaloid under normal cultural conditions. ORCA3 overexpression induces tryptamine, but artificial feeding of the cell lines with the terpenoid precursor loganin is necessary to produce the downstream TIAs. Additionally, biosynthesis of vindoline and dimeric alkaloids vincristine and vinblastine do not occur in the cell lines (van der Fits and Memelink 2000; Zhang et al. 2011). However, it has been demonstrated that cambial meristematic cell cultures of C. roseus can overcome some obstacles of traditional suspension cells, allowing the accumulation of the downstream TIAs, including vindoline, vinblastine, and vincristine (Moon et al. 2015, 2018), making meristematic cell culture a promising platform for TIA engineering. In addition, young C. roseus seedlings (Weaver et al. 2014; Mortensen et al. 2019), leaves (Raina et al. 2012; Sharma et al. 2018a), and flower petals (Schweizer et al. 2018) have also been used for transient overexpression of genes to reprogram both upstream and downstream metabolism.
Heterologous systems, such as yeast and tobacco plants, have also been successfully used to produce several therapeutic metabolites, such as artemisinin from Artemisia annua (Farhi et al. 2011), taxadine from Taxus spp. (Li et al. 2019b), noscapine from Papaver somniferum (Li et al. 2018; Li and Smolke 2016), cannabinoids from Cannabis sativa (Luo et al. 2019), and certain intermediates of TIAs (Miettinen et al. 2014; Qu et al. 2015). The iridoid and indole branch of the TIA pathway has been reconstructed in Nicotiana benthamiana to produce strictosidine (Miettinen et al. 2014), whereas the yeast cells expressing seven-step vindoline pathway are able to produce vindorosine and vindoline (Qu et al. 2015). However, the heterologous systems come with various limitations. The TIA pathway requires more than 30 enzymes in different cellular compartments. Engineering the whole pathway in a heterologous system is therefore cumbersome. The other drawback is the limitation or absence of precursors, which requires introduction of additional genes or precursor feeding to overcome. For instance, production of strictosidine in N. benthamiana leaves requires two additional enzymes, geranyl diphosphate synthase and geraniol synthase, to boost the precursors as well as supplementation of iridoid intermediates (Miettinen et al. 2014). Similarly, tobacco cell suspension culture overexpressing TDC and STR produces strictosidine only after feeding with secologanin (Hallard et al. 1997). Vindoline and vindorosine are produced in yeast cells expressing the seven vindoline pathway genes upon feeding with tabersonine (Qu et al. 2015). Moreover, some downstream TIAs are highly cytotoxic, C. roseus has evolved spatial separation of specific intermediates and transporters for intracellular transport and secretion. We thus argue that a homologous system, such as meristematic cells, young seedlings, hairy roots, or transgenic C. roseus plants, is more suitable for TIA bioengineering.
Technologies and Tools
Gene overexpression and RNAi-mediated silencing are widely used for studying metabolic pathways in plants, including C. roseus (Zhao and Verpoorte 2007; Jaggi et al. 2011; Paul et al. 2017, 2020; Liu et al. 2019; Patra et al. 2018; Suttipanta et al. 2011). Virus-induced gene silencing (VIGS) has emerged as an effective tool to study the regulation of TIA biosynthesis in C. roseus leaves and flowers (Liscombe and O’Connor 2011; Sung et al. 2014; Liu et al. 2019; Patra et al. 2018, 2021). Recently, an improved C. roseus VIGS method has been developed, in which the target gene and the visual marker gene have been incorporated in the same plasmid to successfully identify the silenced tissues in planta (Yamamoto et al. 2021). Furthermore, the generation of stable transgenic C. roseus plants have also been reported (Sharma et al. 2018b; Pan et al. 2012; Wang et al. 2012). The reproducible generation of stable transgenic plants will enable the in planta bioengineering by targeting upstream, midstream, and downstream pathway genes using overexpression, RNAi, and genome-editing (e.g., using CRISPR-Cas9).
TFs are attractive engineering tools as they regulate a subset or all genes in a metabolic pathway. TFs alone, or in combination with key enzymes, have been used to engineer TIA pathway with various degrees of success (Sharma et al. 2020; Pan et al. 2012; Wang et al. 2010). Compared to using individual TFs, combined expression of three TFs ORCA3, BIS1, and a mutant MYC2 significantly upregulates TIA pathway gene expression and increases TIA accumulation in C. roseus flower petals (Schweizer et al. 2018). However, combined overexpression of the three TFs has no effect on downstream TIAs such as vindoline. Although studies on the influence of environmental factors on TIA biosynthesis are limited to a few pathway genes and regulators, they provide important information on the changes in gene expression profiles and TIA accumulation. Additionally, several biotic and abiotic factors have broad effects to TIA biosynthesis, not only to up- and mid-stream metabolites, but also to downstream TIAs, e.g., vindoline. In the following section, we discuss several strategies used previously for engineering TIA biosynthesis. We also describe how the environmental factor-responsive genes can be used as tools to boost TIA production using similar strategies.
Engineering to increase the upstream TIA precursors
Genes encoding several rate-limiting enzymes and TFs, either alone or in combination, have been used to increase the accumulation of upstream metabolites in C. roseus hairy roots or suspension cells. Overexpression of STR in suspension cells greatly induced the accumulation of ajmalicine, serpentine, catharanthine, and tabersonine (Canel et al. 1998). Similarly, combined overexpression of TDC and an Arabidopsis anthanilate synthase (ASα) in hairy roots enhanced the production of tryptamine and serpentine (Hughes et al. 2004). Co-expression of G10H and ORCA3, either in hairy roots or transgenic plants, improved the production of TIAs (Wang et al. 2010; Pan et al. 2012). In addition to using pathway enzyme genes, overexpression of TFs, such as ORCA4 or ORCA5, in hairy roots significantly induced the accumulation of ajmalicine, catharanthine, and tabersonine (Paul et al. 2017, 2020). Although the effects of environmental factors on regulatory genes have not been well studied, the expression of many key upstream pathway genes, such as TDC, STR, and G10H, are altered by environmental factors, such as drought, salt, low temperature, and UV, leading to change in TIA accumulation. These findings suggest that increasing upstream precursors using the key pathway genes or the TFs regulating them will lead to increase of TIA accumulation.
Pushing the metabolic flux towards downstream
Manipulation of the upstream pathway genes can push the metabolite flux to downstream. For instance, transient overexpression of TDC and STR in C. roseus leaves induced expression of downstream pathway genes, including DAT and PRX1, and increases the production of vindoline and vinblastine (Sharma et al. 2018a). Co-expression of ORCA3 and G10H in C. roseus plants not only increased the accumulation of the midstream metabolites ajmalicine and catharanthine, but also the downstream vindoline (Pan et al. 2012). Expression of TDC, STR, D4H, and DAT was altered by various external factors, such as UV and herbivory, leading to the increase in midstream and downstream TIAs, suggesting their potentials for increasing TIA production.
Pulling the metabolic flux to downstream
Metabolic flux can be pulled towards downstream by manipulating the downstream TIA biosynthetic steps. Overexpression of the key vindoline pathway gene DAT in C. roseus plants increased the production of vindoline (Wang et al. 2012). Transient overexpression of the transcription activator CrGATA1 in seedlings improved vindoline accumulation (Liu et al. 2019). Knocking down the expression of the transcription repressor CrPIF1 in leaves by VIGS also improved vindoline accumulation (Liu et al. 2019). Expression of CrGATA1 and other vindoline pathway genes is affected by light. The light-induced vindoline and the dimeric TIAs, such as vinblastine and vincristine, are accumulated in aerial parts of the plants. The genes encoding downstream enzymes, such as D4H and DAT, or TFs, such as CrGATA1, may be co-overexpressed with PRX1 either in seedlings or transgenic plants to boost TIA production. Alternatively, the meristematic cell culture, that is capable of producing the dimeric TIAs, can be used to test this strategy.
Increasing the downstream TIAs through a push-and-pull strategy
The production of downstream TIAs can be maximized through combination of push and pull strategies. Overexpression of the transcriptional activator CrERF5 in C. roseus petals induces the expression of the upstream TDC and STR, as well as the downstream D4H and PRX1 (Pan et al. 2019). VIGS of the transcription repressor CR1 in C. roseus leaves also upregulates TDC, STR, DAT, and PRX1 (Liu et al. 2017a). Similarly, transient overexpression of the kinase CrMAPK3 in C. roseus leaves upregulates the expression of TDC, STR, D4H, and DAT (Raina et al. 2012). However, it is unclear whether CrERF5 or CR1 directly regulates both upstream and downstream genes, but rather, regulates only one subset of the pathway genes such that the following metabolite flux affects the other subset of the genes. Additionally, whether the expression of these known regulatory genes is affected by environmental factors requires further study. Therefore, detailed analysis of spatio-temporal expression profiles of known regulators in response to different environmental stimuli will provide additional tools for TIA metabolic engineering. Expression of many upstream and downstream TIA pathway genes, such as STR, TDC, G10H, DAT and D4H, is altered by UV, salt, high temperature, and herbivory, leading to the increase in dimeric alkaloid and its precursors such as vindoline and catharanthine. Therefore, combined overexpression of upstream and downstream pathway genes responsive to environmental factors will potentially boost TIA production.
Regulatory factors associated with UV-B signal transduction are well characterized in Arabidopsis (Morales et al. 2013; Rizzini et al. 2011). In Arabidopsis, the UV-B receptor UVR8 regulates expression of the genes involved in UV protection and defense response, as well as biosynthesis and signaling of JA and SA. The UV receptor and other regulatory factors in the UV signaling pathway are conserved across plant species (Tossi et al. 2019), and UV induces the accumulation of both upstream and downstream TIAs. Therefore, the signaling components associated with the UV-B pathway can be potential targets to increase TIAs in C. roseus.
Conclusions
Biosynthesis of many specialized metabolites is affected by environmental factors (Li et al. 2020; Yang et al. 2018). One notable example is anthocyanins often found in fruits, vegetable and flowers (Maier et al. 2013; Plunkett et al. 2019; Xie et al. 2012). The accumulation of other specialized metabolites, such as artemisinin, is also affected by low light, temperature, and UV (Xiang et al. 2019; Hao et al. 2019; Pan et al. 2014). Systematic studies on the influence of environmental factors led to the identification key regulatory genes and the underlying molecular mechanisms governing biosynthesis of these metabolites. The anticancer drugs vinblastine and vincristine are in demand but produced in extremely low quantities in C. roseus leaves. Attempts to increase TIAs through metabolic engineering met with various degrees of success. Studies on environmental factors clearly show that drought, salt, light, and temperature affect the production of both upstream and downstream TIAs in C. roseus. The increase or decrease of TIA accumulation in response to environmental factors is likely a consequence of the changes in the expression of pathway genes, regulators, and signal transduction components, such as protein kinases. Gene regulation of TIA biosynthesis is highly complex. However, a comprehensive mechanistic study on how environmental factors regulate pathway gene expression to affect TIA biosynthesis is lacking. In the past few years, a number of genes encoding key pathway enzymes, kinases, and regulators in the TIA pathway have been identified and characterized. Transporters play key roles in the intracellular transport of TIA intermediates. However, the influence of environmental factors on TIA transporters and the newly identified genes have not been studied. Moreover, many repressors involved in the regulation of the TIA pathway have been discovered recently (Shoji and Yuan 2021; Patra et al. 2018; Pauw et al. 2004; Sui et al. 2018). The repressors, working in concert with the activators, enable C. roseus to dial the amplitude of TIA biosynthesis. Expression profiles of these repressors in response to environmental factors will provide important insights on TIA regulation. The past engineering approaches heavily rely on overexpression of positive regulators and key enzymes. Overexpression of a positive regulator while knockdown or knockout of a repressor could be an alternative strategy to engineer TIA biosynthesis. RNA-sequencing has emerged as a powerful tool to study transcriptomic landscape in response to any biotic or abiotic factors. Transcriptomic analyses in response light, JA, and UV provided important information on factors involved in artemisinin biosynthesis (Hao et al. 2017; Pan et al. 2014). C. roseus transcriptomic analyses also led to the identification of new pathway genes and regulators. The majority of published studies focus on individual environmental factor on TIA accumulation. However, plants are subject to many biotic and abiotic stress factors in a natural environment. “Stress combination transcriptomics” attempt to dissect the plant responses to different combinations of biotic and/or abiotic stresses (Zandalinas et al. 2020). Study on combined effects of environmental factors on specialized metabolism is still lacking. Generation and analyses of transcriptomes of C. roseus in response to different environmental factors will allow further elucidation of the regulation of TIA pathway, thus generating potential candidates for metabolic engineering.
Acknowledgements
Y.Z is supported by a scholarship from the Department of Plant and Soil Sciences, and Kentucky Tobacco Research and Development Center, University of Kentucky.
Author contributions
Y.L., B.P., S.K.S., Y.Z, Y.L., Y.W. S.P., L.Y wrote the paper.
Funding
This work is partially funded by the National Key R&D Projects from the Ministry of Science and Technology of China, 2019YFC1711102 and 2019YFC1711104, to Y.L.
Declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yan Zhou, Email: yzh477@uky.edu.
Yongqing Li, Email: liyongqing@scbg.ac.cn.
Ying Wang, Email: yingwang@scib.ac.cn.
Sitakanta Pattanaik, Email: spatt2@uky.edu.
Ling Yuan, Email: lyuan3@uky.edu.
References
- Ababaf M, Omidi H, Bakhshadeh A. Changes in antioxidant enzymes activities and alkaloid amount of Catharanthus roseus in response to plant growth regulators under drought condition. Ind Crop Prod. 2021;167:113505. doi: 10.1016/j.indcrop.2021.113505. [DOI] [Google Scholar]
- Ahmadzadeh M, Keshtkar A, Moslemkhany K, Ahmadzadeh M (2020) Evaluation of water deficite stress and plant growth-promoting rhizobacteria effect on some of morphological traits and expression level of TDC and STR at the root of Catharanthus roseus. J Plant Res. https://plant.ijbio.ir/article_1768.html
- Amirjani MR. Effects of drought stress on the alkaloid contents and growth parameters of Catharanthus roseus. J Agric Biol Sci. 2013;8(11):745–750. [Google Scholar]
- Binder BYK, Peebles CAM, Shanks JV, San K-Y. The effects of UV-B stress on the production of terpenoid indole alkaloids in Catharanthus roseus hairy roots. Biotechnol Prog. 2009;25(3):861–865. doi: 10.1002/btpr.97. [DOI] [PubMed] [Google Scholar]
- Canel C, Lopes-Cardoso MI, Whitmer S, van der Fits L, Pasquali G, van der Heijden R, Hoge JHC, Verpoorte R. Effects of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta. 1998;205(3):414–419. doi: 10.1007/s004250050338. [DOI] [PubMed] [Google Scholar]
- Carqueijeiro I, Brown S, Chung K, Dang TT, Walia M, Besseau S, Duge de Bernonville T, Oudin A, Lanoue A, Billet K, Munsch T, Koudounas K, Melin C, Godon C, Razafimandimby B, de Craene JO, Glevarec G, Marc J, Giglioli-Guivarc’h N, Clastre M, St-Pierre B, Papon N, Andrade RB, O’Connor SE, Courdavault V. Two tabersonine 6,7-epoxidases initiate lochnericine-derived alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 2018;177(4):1473–1486. doi: 10.1104/pp.18.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carqueijeiro I, Duge de Bernonville T, Lanoue A, Dang TT, Teijaro CN, Paetz C, Billet K, Mosquera A, Oudin A, Besseau S, Papon N, Glevarec G, Atehortua L, Clastre M, Giglioli-Guivarc’h N, Schneider B, St-Pierre B, Andrade RB, O’Connor SE, Courdavault V, A BAHD acyltransferase catalyzing 19-O-acetylation of tabersonine derivatives in roots of Catharanthus roseus enables combinatorial synthesis of monoterpene indole alkaloids. Plant J. 2018;94(3):469–484. doi: 10.1111/tpj.13868. [DOI] [PubMed] [Google Scholar]
- Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol. 1999;70(1):1–9. doi: 10.1111/j.1751-1097.1999.tb01944.x. [DOI] [Google Scholar]
- Chen Q, Chen Z, Lu L, Jin H, Sun L, Yu Q, Xu H, Yang F, Fu M, Li S. Interaction between abscisic acid and nitric oxide in PB90-induced catharanthine biosynthesis of Catharanthus roseus cell suspension cultures. Biotechnol Prog. 2013;29(4):994–1001. doi: 10.1002/btpr.1738. [DOI] [PubMed] [Google Scholar]
- Chen Q, Lu X, Guo X, Pan Y, Yu B, Tang Z, Guo Q. Differential responses to Cd stress induced by exogenous application of Cu, Zn or Ca in the medicinal plant Catharanthus roseus. Ecotoxicol Environ Saf. 2018;157:266–275. doi: 10.1016/j.ecoenv.2018.03.055. [DOI] [PubMed] [Google Scholar]
- Cohen SD, Kennedy JA. Plant metabolism and the environment: implications for managing phenolics. Crit Rev Food Sci Nutr. 2010;50(7):620–643. doi: 10.1080/10408390802603441. [DOI] [PubMed] [Google Scholar]
- Courdavault V, Papon N, Clastre M, Giglioli-Guivarc’h N, St-Pierre B, Burlat V. A look inside an alkaloid multisite plant: the Catharanthus logistics. Curr Opin Plant Biol. 2014;19:43–50. doi: 10.1016/j.pbi.2014.03.010. [DOI] [PubMed] [Google Scholar]
- De Bernonville TD, Carqueijeiro I, Lanoue A, Lafontaine F, Bel PS, Liesecke F, Musset K, Oudin A, Glévarec G, Pichon O. Folivory elicits a strong defense reaction in Catharanthus roseus: metabolomic and transcriptomic analyses reveal distinct local and systemic responses. Sci Rep. 2017;7(1):1–14. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carolis E, Chan F, Balsevich J, De Luca V. Isolation and characterization of a 2-oxoglutarate dependent dioxygenase involved in the second-to-last step in vindoline biosynthesis. Plant Physiol. 1990;94(3):1323–1329. doi: 10.1104/pp.94.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca V, Balsevich J, Tyler R, Eilert U, Panchuk B, Kurz W. Biosynthesis of indole alkaloids: developmental regulation of the biosynthetic pathway from tabersonine to vindoline in Catharanthus roseus. J Plant Physiol. 1986;125(1–2):147–156. doi: 10.1016/S0176-1617(86)80252-8. [DOI] [Google Scholar]
- De Luca V, Salim V, Thamm A, Masada SA, Yu F. Making iridoids/secoiridoids and monoterpenoid indole alkaloids: progress on pathway elucidation. Curr Opin Plant Biol. 2014;19:35–42. doi: 10.1016/j.pbi.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Dong X. SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol. 1998;1(4):316–323. doi: 10.1016/1369-5266(88)80053-0. [DOI] [PubMed] [Google Scholar]
- Dutta A, Sen J, Deswal R. Downregulation of terpenoid indole alkaloid biosynthetic pathway by low temperature and cloning of a AP2 type C-repeat binding factor (CBF) from Catharanthus roseus (L). G. Don. Plant Cell Rep. 2007;26(10):1869–1878. doi: 10.1007/s00299-007-0383-y. [DOI] [PubMed] [Google Scholar]
- Dutta A, Sen J, Deswal R. New evidences about strictosidine synthase (Str) regulation by salinity, cold stress and nitric oxide in Catharanthus roseus. J Plant Biochem Biotechnol. 2013;22(1):124–131. doi: 10.1007/s13562-012-0118-1. [DOI] [Google Scholar]
- El-Sayed M, Verpoorte R. Growth, metabolic profiling and enzymes activities of Catharanthus roseus seedlings treated with plant growth regulators. Plant Growth Regul. 2004;44(1):53–58. doi: 10.1007/s10725-004-2604-5. [DOI] [Google Scholar]
- Farhi M, Marhevka E, Ben-Ari J, Algamas-Dimantov A, Liang Z, Zeevi V, Edelbaum O, Spitzer-Rimon B, Abeliovich H, Schwartz B, Tzfira T, Vainstein A. Generation of the potent anti-malarial drug artemisinin in tobacco. Nat Biotechnol. 2011;29(12):1072–1074. doi: 10.1038/nbt.2054. [DOI] [PubMed] [Google Scholar]
- Fatima S, Mujib A, Tonk DJPC, Tissue, Culture O. NaCl amendment improves vinblastine and vincristine synthesis in Catharanthus roseus: a case of stress signalling as evidenced by antioxidant enzymes activities. Plant Cell Tissue Organ Cult. 2015;121(2):445–458. doi: 10.1007/s11240-015-0715-5. [DOI] [Google Scholar]
- Fouad AS, Hafez RM. Effect of cobalt nanoparticles and cobalt ions on alkaloids production and expression of CrMPK3 gene in Catharanthus roseus suspension cultures. Cell Mol Biol (Noisy-le-grand) 2018;64(12):62–69. doi: 10.14715/cmb/2018.64.12.13. [DOI] [PubMed] [Google Scholar]
- Frohnmeyer H, Staiger D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003;133(4):1420–1428. doi: 10.1104/pp.103.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholamhosseinpour Z, Hemati K, Dorodian H, Bashiri-Sadr Z. Effect of nitrogen fertilizer on yield and amount of alkaloids in periwinkle and determination of vinblastine and vincristine by HPLC and TLC. Plant Sci Res. 2011;3(2):4–9. doi: 10.3923/psres.2011.4.9. [DOI] [Google Scholar]
- Giddings LA, Liscombe DK, Hamilton JP, Childs KL, DellaPenna D, Buell CR, O’Connor SE. A stereoselective hydroxylation step of alkaloid biosynthesis by a unique cytochrome P450 in Catharanthus roseus. J Biol Chem. 2011;286(19):16751–16757. doi: 10.1074/jbc.M111.225383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirimand G, Courdavault V, Lanoue A, Mahroug S, Guihur A, Blanc N, Giglioli-Guivarc'h N, St-Pierre B, Burlat V. Strictosidine activation in apocynaceae: towards a “nuclear time bomb”? BMC Plant Biol. 2010;10:182. doi: 10.1186/1471-2229-10-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X-r, Yang L, Yu J-h, Tang Z-h, Zu Y-g. Alkaloid variations in Catharanthus roseus seedlings treated by different temperatures in short term and long term. J For Res. 2007;18(4):313–315. doi: 10.1007/s11676-007-0063-3. [DOI] [Google Scholar]
- Guo X-R, Chang B-W, Zu Y-G, Tang Z-H. The impacts of increased nitrate supply on Catharanthus roseus growth and alkaloid accumulations under ultraviolet-B stress. J Plant Interact. 2014;9(1):640–646. doi: 10.1080/17429145.2014.886728. [DOI] [Google Scholar]
- Hallard D, van der Heijden R, Verpoorte R, Cardoso MIL, Pasquali G, Memelink J, Hoge JHC. Suspension cultured transgenic cells of Nicotiana tabacum expressing tryptophan decarboxylase and strictosidine synthase cDNAs from Catharanthus roseus produce strictosidine upon secologanin feeding. Plant Cell Rep. 1997;17(1):50–54. doi: 10.1007/s002990050350. [DOI] [PubMed] [Google Scholar]
- Han L, Li GJ, Yang KY, Mao G, Wang R, Liu Y, Zhang S. Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea‐induced ethylene production in Arabidopsis. Plant J. 2010;64(1):114–127. doi: 10.1111/j.1365-313X.2010.04318.x. [DOI] [PubMed] [Google Scholar]
- Hao X, Zhong Y, Fu X, Lv Z, Shen Q, Yan T, Shi P, Ma Y, Chen M, Lv X, Wu Z, Zhao J, Sun X, Li L, Tang K. Transcriptome analysis of genes associated with the artemisinin biosynthesis by jasmonic acid treatment under the Light in Artemisia annua. Front Plant Sci. 2017;8:971. doi: 10.3389/fpls.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X, Zhong Y, Ni Tzmann HW, Fu X, Yan T, Shen Q, Chen M, Ma Y, Zhao J, Osbourn A, Li L, Tang K. Light-induced artemisinin biosynthesis is regulated by the bZIP transcription factor AaHY5 in Artemisia annua. Plant Cell Physiol. 2019;60(8):1747–1760. doi: 10.1093/pcp/pcz084. [DOI] [PubMed] [Google Scholar]
- Hughes EH, Shanks JV. Metabolic engineering of plants for alkaloid production. Metab Eng. 2002;4(1):41–48. doi: 10.1006/mben.2001.0205. [DOI] [PubMed] [Google Scholar]
- Hughes EH, Hong S-B, Gibson SI, Shanks JV, San K-Y. Metabolic engineering of the indole pathway in Catharanthus roseus hairy roots and increased accumulation of tryptamine and serpentine. Metab Eng. 2004;6(4):268–276. doi: 10.1016/j.ymben.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Idrees M, Naeem M, Aftab T, Khan MMA. Salicylic acid mitigates salinity stress by improving antioxidant defence system and enhances vincristine and vinblastine alkaloids production in periwinkle [Catharanthus roseus (L.) G. Don] Acta Physiol Plant. 2011;33(3):987–999. doi: 10.1007/s11738-010-0631-6. [DOI] [Google Scholar]
- Jaggi M, Kumar S, Sinha AK. Overexpression of an apoplastic peroxidase gene CrPrx in transgenic hairy root lines of Catharanthus roseus. Appl Microbiol Biotechnol. 2011;90(3):1005–1016. doi: 10.1007/s00253-011-3131-8. [DOI] [PubMed] [Google Scholar]
- Jaleel CA, Gopi R, Kishorekumar A, Manivannan P, Sankar B, Panneerselvam R. Interactive effects of triadimefon and salt stress on antioxidative status and ajmalicine accumulation in Catharanthus roseus. Acta Physiol Plant. 2008;30(3):287. doi: 10.1007/s11738-007-0119-1. [DOI] [Google Scholar]
- Jaleel CA, Sankar B, Murali P, Gomathinayagam M, Lakshmanan G, Panneerselvam R. Water deficit stress effects on reactive oxygen metabolism in Catharanthus roseus; impacts on ajmalicine accumulation. Colloids Surf B. 2008;62(1):105–111. doi: 10.1016/j.colsurfb.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Jaleel CA, Sankar B, Sridharan R, Panneerselvam R. Soil salinity alters growth, chlorophyll content, and secondary metabolite accumulation in Catharanthus roseus. Turk J Biol. 2008;32(2):79–83. [Google Scholar]
- Kim S, Hwang G, Lee S, Zhu J-Y, Paik I, Nguyen TT, Kim J, Oh E. High ambient temperature represses anthocyanin biosynthesis through degradation of HY5. Front Plant Sci. 2017;8:1787. doi: 10.3389/fpls.2017.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Fuller VL, Pollier J, Van Moerkercke A, Schweizer F, Payne R, Colinas M, O’Connor SE, Goossens A, Halkier BA. Identification of iridoid glucoside transporters in Catharanthus roseus. Plant Cell Physiol. 2017;58(9):1507–1518. doi: 10.1093/pcp/pcx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Smolke CD. Engineering biosynthesis of the anticancer alkaloid noscapine in yeast. Nat Commun. 2016;7:12137. doi: 10.1038/ncomms12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ou-Lee T-M, Raba R, Amundson RG, Last RL. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell. 1993;5(2):171–179. doi: 10.2307/3869583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Leopold AL, Sander GW, Shanks JV, Zhao L, Gibson SI. The ORCA2 transcription factor plays a key role in regulation of the terpenoid indole alkaloid pathway. BMC Plant Biol. 2013;13(1):1–17. doi: 10.1186/1471-2229-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Wang W, Gao J, Yin K, Wang R, Wang C, Petersen M, Mundy J, Qiu J-L. MYB75 phosphorylation by MPK4 is required for light-induced anthocyanin accumulation in Arabidopsis. Plant Cell. 2016;28(11):2866–2883. doi: 10.1105/tpc.16.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ding Y, Shi Y, Zhang X, Zhang S, Gong Z, Yang S. MPK3-and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev Cell. 2017;43(5):630–642. doi: 10.1016/j.devcel.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Thodey K, Trenchard I, Cravens A, Smolke CD. Complete biosynthesis of noscapine and halogenated alkaloids in yeast. Proc Natl Acad Sci USA. 2018;115(17):E3922–E3931. doi: 10.1073/pnas.1721469115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Fan R, Guo S, Wang P, Zhu X, Fan Y, Chen Y, He K, Kumar A, Shi J. The Arabidopsis MYB transcription factor, MYB111 modulates salt responses by regulating flavonoid biosynthesis. Environ Exp Bot. 2019;166:103807. doi: 10.1016/j.envexpbot.2019.103807. [DOI] [Google Scholar]
- Li J, Mutanda I, Wang K, Yang L, Wang J, Wang Y. Chloroplastic metabolic engineering coupled with isoprenoid pool enhancement for committed taxanes biosynthesis in Nicotiana benthamiana. Nat Commun. 2019;10(1):4850. doi: 10.1038/s41467-019-12879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol Biochem. 2020;148:80–89. doi: 10.1016/j.plaphy.2020.01.006. [DOI] [PubMed] [Google Scholar]
- Liscombe DK, O’Connor SE. A virus-induced gene silencing approach to understanding alkaloid metabolism in Catharanthus roseus. Phytochemistry. 2011;72(16):1969–1977. doi: 10.1016/j.phytochem.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang Y, Wang J, Li P, Zhao C, Chen Y, Bi Y. Phytochrome-interacting factors PIF4 and PIF5 negatively regulate anthocyanin biosynthesis under red light in Arabidopsis seedlings. Plant Sci. 2015;238:64–72. doi: 10.1016/j.plantsci.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao F, Ren J, Lu X, Ren G, Wang R. A novel AP2/ERF transcription factor CR1 regulates the accumulation of vindoline and serpentine in Catharanthus roseus. Front Plant Sci. 2017;8:2082. doi: 10.3389/fpls.2017.02082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Meng Q, Duan X, Zhang Z, Li D. Effects of PEG-induced drought stress on regulation of indole alkaloid biosynthesis in Catharanthus roseus. J Plant Interact. 2017;12(1):87–91. doi: 10.1080/17429145.2017.1293852. [DOI] [Google Scholar]
- Liu Y, Patra B, Pattanaik S, Wang Y, Yuan L. GATA and phytochrome interacting factor transcription factors regulate light-induced vindoline biosynthesis in Catharanthus roseus. Plant Physiol. 2019;180(3):1336–1350. doi: 10.1104/pp.19.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Reiter MA, d’Espaux L, Wong J, Denby CM, Lechner A, Zhang Y, Grzybowski AT, Harth S, Lin W, Lee H, Yu C, Shin J, Deng K, Benites VT, Wang G, Baidoo EEK, Chen Y, Dev I, Petzold CJ, Keasling JD. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature. 2019;567(7746):123–126. doi: 10.1038/s41586-019-0978-9. [DOI] [PubMed] [Google Scholar]
- Maier A, Schrader A, Kokkelink L, Falke C, Welter B, Iniesto E, Rubio V, Uhrig JF, Hulskamp M, Hoecker U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J. 2013;74(4):638–651. doi: 10.1111/tpj.12153. [DOI] [PubMed] [Google Scholar]
- Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell. 2011;23(4):1639–1653. doi: 10.1105/tpc.111.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqsood M, Abdul M. Yeast extract elicitation increases vinblastine and vincristine yield in protoplast derived tissues and plantlets in Catharanthus roseus. Revista Brasileira de Farmacognosia. 2017;27(5):549–556. doi: 10.1016/j.bjp.2017.05.008. [DOI] [Google Scholar]
- Mendonça Freitas MS, Gama MC, Monnerat PH, De Carvalho AJC, Lima TC, Vieira IJC. Induced nutrient deficiencies in Catharanthus roseus impact ajmalicine bioproduction. J Plant Nutr. 2016;39(6):835–841. doi: 10.1080/01904167.2015.1047524. [DOI] [Google Scholar]
- Meng X, Zhang S. MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol. 2013;51:245–266. doi: 10.1146/annurev-phyto-082712-102314. [DOI] [PubMed] [Google Scholar]
- Menke FL, Champion A, Kijne JW, Memelink J. A novel jasmonate-and elicitor‐responsive element in the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate‐and elicitor‐inducible AP2‐domain transcription factor, ORCA2. EMBO J. 1999;18(16):4455–4463. doi: 10.1093/emboj/18.16.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen K, Dong L, Navrot N, Schneider T, Burlat V, Pollier J, Woittiez L, Van Der Krol S, Lugan R, Ilc T. The seco-iridoid pathway from Catharanthus roseus. Nat Commun. 2014;5(1):1–12. doi: 10.1038/ncomms4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhaberi A, Ahmadi J, Mafakheri S. The expression profile of D4H and DAT genes in Catharanthus roseus in response to drought, salinity and salicylic acid. Iranian J Genet Plant Breed. 2013;2:38–46. [Google Scholar]
- Moon SH, Venkatesh J, Yu J-W, Park SW. Differential induction of meristematic stem cells of Catharanthus roseus and their characterization. Comptes rendus biologies. 2015;338(11):745–756. doi: 10.1016/j.crvi.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Moon SH, Pandurangan M, Kim DH, Venkatesh J, Patel RV, Mistry BM. A rich source of potential bioactive compounds with anticancer activities by Catharanthus roseus cambium meristematic stem cell cultures. J Ethnopharmacol. 2018;217:107–117. doi: 10.1016/j.jep.2018.02.021. [DOI] [PubMed] [Google Scholar]
- Morales LO, Brosche M, Vainonen J, Jenkins GI, Wargent JJ, Sipari N, Strid A, Lindfors AV, Tegelberg R, Aphalo PJ. Multiple roles for UV RESISTANCE LOCUS8 in regulating gene expression and metabolite accumulation in Arabidopsis under solar ultraviolet radiation. Plant Physiol. 2013;161(2):744–759. doi: 10.1104/pp.112.211375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JA, Shanks JV. Determination of metabolic rate-limitations by precursor feeding in Catharanthus roseus hairy root cultures. J Biotechnol. 2000;79(2):137–145. doi: 10.1016/S0168-1656(00)00221-2. [DOI] [PubMed] [Google Scholar]
- Mortensen S, Bernal-Franco D, Cole LF, Sathitloetsakun S, Cram EJ, Lee-Parsons CW. EASI transformation: an efficient transient expression method for analyzing gene function in Catharanthus roseus seedlings. Front Plant Sci. 2019;10:755. doi: 10.3389/fpls.2019.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namdeo A, Patil S, Fulzele DPJBp. Influence of fungal elicitors on production of ajmalicine by cell cultures of Catharanthus roseus. Biotechnol Prog. 2002;18(1):159–162. doi: 10.1021/bp0101280. [DOI] [PubMed] [Google Scholar]
- Nazari M, Zarinkamar F. Ultraviolet-B induced changes in Mentha aquatica (a medicinal plant) at early and late vegetative growth stages: investigations at molecular and genetic levels. Ind Crop Prod. 2020;154:112618. doi: 10.1016/j.indcrop.2020.112618. [DOI] [Google Scholar]
- Osman ME, Elfeky SS, El-Soud KA, Hasan AM. Response of Catharanthus roseus shoots to salinity and drought in relation to vincristine alkaloid content. Asian J Plant Sci. 2007;6:1223–1228. doi: 10.3923/ajps.2007.1223.1228. [DOI] [Google Scholar]
- Ouwerkerk P, Trimborn T, Hilliou F, Memelink J. Nuclear factors GT-1 and 3AF1 interact with multiple sequences within the promoter of the Tdc gene from Madagascar periwinkle: GT-1 is involved in UV light-induced expression. Mol Gen Genet. 1999;261(4):610–622. doi: 10.1007/s004380050003. [DOI] [PubMed] [Google Scholar]
- Ouwerkerk PB, Hallard D, Verpoorte R, Memelink J. Identification of UV-B light-responsive regions in the promoter of the tryptophan decarboxylase gene from Catharanthus roseus. Plant Mol Biol. 1999;41(4):491–503. doi: 10.1023/A:1006321100550. [DOI] [PubMed] [Google Scholar]
- Pan Q, Chen Y, Wang Q, Yuan F, Xing S, Tian Y, Zhao J, Sun X, Tang KJPgr, Effect of plant growth regulators on the biosynthesis of vinblastine, vindoline and catharanthine in Catharanthus roseus. Plant Growth Regul. 2010;60(2):133–141. doi: 10.1007/s10725-009-9429-1. [DOI] [Google Scholar]
- Pan Q, Wang Q, Yuan F, Xing S, Zhao J, Choi YH, Verpoorte R, Tian Y, Wang G, Tang K. Overexpression of ORCA3 and G10H in Catharanthus roseus plants regulated alkaloid biosynthesis and metabolism revealed by NMR-metabolomics. PLoS ONE. 2012;7(8):e43038. doi: 10.1371/journal.pone.0043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WS, Zheng LP, Tian H, Li WY, Wang JW. Transcriptome responses involved in artemisinin production in Artemisia annua L. under UV-B radiation. J Photochem Photobiol B. 2014;140:292–300. doi: 10.1016/j.jphotobiol.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Pan Y-J, Liu J, Guo X-R, Zu Y-G, Tang Z-H. Gene transcript profiles of the TIA biosynthetic pathway in response to ethylene and copper reveal their interactive role in modulating TIA biosynthesis in Catharanthus roseus. Protoplasma. 2015;252:813–824. doi: 10.1007/s00709-014-0718-9. [DOI] [PubMed] [Google Scholar]
- Pan Q, Wang C, Xiong Z, Wang H, Fu X, Shen Q, Peng B, Ma Y, Sun X, Tang K. CrERF5, an AP2/ERF transcription factor, positively regulates the biosynthesis of bisindole alkaloids and their precursors in Catharanthus roseus. Front Plant Sci. 2019;10:931. doi: 10.3389/fpls.2019.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Kazachkova Y, Aharoni A. Catch-22 in specialized metabolism: balancing defense and growth. J Exp Bot. 2021 doi: 10.1093/jxb/erab348. [DOI] [PubMed] [Google Scholar]
- Pandey SS, Singh S, Babu CV, Shanker K, Srivastava N, Shukla AK, Kalra A. Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci Rep. 2016;6(1):1–14. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra B, Schluttenhofer C, Wu Y, Pattanaik S, Yuan L. Transcriptional regulation of secondary metabolite biosynthesis in plants. Biochim Biophys Acta. 2013;1829(11):1236–1247. doi: 10.1016/j.bbagrm.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Patra B, Pattanaik S, Schluttenhofer C, Yuan L. A network of jasmonate-responsive bHLH factors modulate monoterpenoid indole alkaloid biosynthesis in Catharanthus roseus. New Phytol. 2018;217(4):1566–1581. doi: 10.1111/nph.14910. [DOI] [PubMed] [Google Scholar]
- Patra B, Liu Y, Singleton JJ, Singh SK, Pattanaik S, Yuan L (2021) Virus-induced gene silencing as a tool to study regulation of alkaloid biosynthesis in medicinal plants. Methods Mol Biol (in press) [DOI] [PubMed]
- Paul P, Singh SK, Patra B, Sui X, Pattanaik S, Yuan L. A differentially regulated AP2/ERF transcription factor gene cluster acts downstream of a MAP kinase cascade to modulate terpenoid indole alkaloid biosynthesis in Catharanthus roseus. New Phytol. 2017;213(3):1107–1123. doi: 10.1111/nph.14252. [DOI] [PubMed] [Google Scholar]
- Paul P, Singh SK, Patra B, Liu X, Pattanaik S, Yuan L. Mutually regulated AP2/ERF gene clusters modulate biosynthesis of specialized metabolites in plants. Plant Physiol. 2020;182(2):840–856. doi: 10.1104/pp.19.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauw B, Hilliou FA, Martin VS, Chatel G, de Wolf CJ, Champion A, Pré M, van Duijn B, Kijne JW, van der Fits L. Zinc finger proteins act as transcriptional repressors of alkaloid biosynthesis genes in Catharanthus roseus. J Biol Chem. 2004;279(51):52940–52948. doi: 10.1074/jbc.M404391200. [DOI] [PubMed] [Google Scholar]
- Payne RM, Xu D, Foureau E, Teto Carqueijeiro MI, Oudin A, Bernonville TD, Novak V, Burow M, Olsen CE, Jones DM, Tatsis EC, Pendle A, Ann Halkier B, Geu-Flores F, Courdavault V, Nour-Eldin HH, O’Connor SE. An NPF transporter exports a central monoterpene indole alkaloid intermediate from the vacuole. Nat Plants. 2017;3:16208. doi: 10.1038/nplants.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles CA, Hughes EH, Shanks JV, San K-Y. Transcriptional response of the terpenoid indole alkaloid pathway to the overexpression of ORCA3 along with jasmonic acid elicitation of Catharanthus roseus hairy roots over time. Metab Eng. 2009;11(2):76–86. doi: 10.1016/j.ymben.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Plunkett BJ, Henry-Kirk R, Friend A, Diack R, Helbig S, Mouhu K, Tomes S, Dare AP, Espley RV, Putterill J, Allan AC. Apple B-box factors regulate light-responsive anthocyanin biosynthesis genes. Sci Rep. 2019;9(1):17762. doi: 10.1038/s41598-019-54166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Easson ML, Froese J, Simionescu R, Hudlicky T, De Luca V. Completion of the seven-step pathway from tabersonine to the anticancer drug precursor vindoline and its assembly in yeast. Proc Natl Acad Sci. 2015;112(19):6224–6229. doi: 10.1073/pnas.1501821112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Easson ME, Simionescu R, Hajicek J, Thamm AM, Salim V, De Luca V. Solution of the multistep pathway for assembly of corynanthean, strychnos, iboga, and aspidosperma monoterpenoid indole alkaloids from 19E-geissoschizine. Proc Natl Acad Sci. 2018;115(12):3180–3185. doi: 10.1073/pnas.1719979115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Safonova O, De Luca V. Completion of the canonical pathway for assembly of anticancer drugs vincristine/vinblastine in Catharanthus roseus. Plant J. 2019;97(2):257–266. doi: 10.1111/tpj.14111. [DOI] [PubMed] [Google Scholar]
- Rai R, Meena RP, Smita SS, Shukla A, Rai SK, Pandey-Rai S. UV-B and UV-C pre-treatments induce physiological changes and artemisinin biosynthesis in Artemisia annua L.—an antimalarial plant. J Photochem Photobiol B. 2011;105(3):216–225. doi: 10.1016/j.jphotobiol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Rai V, Tandon PK, Khatoon S. Effect of chromium on antioxidant potential of Catharanthus roseus varieties and production of their anticancer alkaloids: vincristine and vinblastine. BioMed Res Int. 2014 doi: 10.1155/2014/934182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina SK, Wankhede DP, Jaggi M, Singh P, Jalmi SK, Raghuram B, Sheikh AH, Sinha AK. CrMPK3, a mitogen activated protein kinase from Catharanthus roseus and its possible role in stress induced biosynthesis of monoterpenoid indole alkaloids. BMC Plant Biol. 2012;12(1):1–13. doi: 10.1186/1471-2229-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina S, Wankhede D, Sinha A. Catharanthus roseus mitogen-activated protein kinase 3 confers UV and heat tolerance to Saccharomyces cerevisiae. Plant Signal Behav. 2013;8(1):e22716. doi: 10.4161/psb.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani S, Chelliah J. UV-B-induced signaling events leading to enhanced-production of catharanthine in Catharanthus roseus cell suspension cultures. BMC Plant Biol. 2007;7(1):61. doi: 10.1186/1471-2229-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani S, Jayabaskaran C. Enhanced catharanthine and vindoline production in suspension cultures of Catharanthus roseus by ultraviolet-B light. J Mol Signal. 2008;3(1):1–6. doi: 10.1186/1750-2187-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y-R, Zhao Q, Yang Y-Y, Zhang T-E, Wang X-F, You C-X, Hao Y-J. The apple 14-3-3 protein MdGRF11 interacts with the BTB protein MdBT2 to regulate nitrate deficiency-induced anthocyanin accumulation. Hort Res. 2021;8(1):1–14. doi: 10.1038/s41438-020-00428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijhwani SK, Shanks JV. Effect of elicitor dosage and exposure time on biosynthesis of indole alkaloids by Catharanthus roseus hairy root cultures. Biotechnol Prog. 1998;14(3):442–449. doi: 10.1021/bp980029v. [DOI] [PubMed] [Google Scholar]
- Rizzini L, Favory JJ, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schafer E, Nagy F, Jenkins GI, Ulm R. Perception of UV-B by the Arabidopsis UVR8 protein. Science. 2011;332(6025):103–106. doi: 10.1126/science.1200660. [DOI] [PubMed] [Google Scholar]
- Roepke J, Salim V, Wu M, Thamm AM, Murata J, Ploss K, Boland W, De Luca V. Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. Proc Natl Acad Sci. 2010;107(34):15287–15292. doi: 10.1073/pnas.0911451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama I, Eliwa N, Mohamed M. Effect of UV-A on vincristine biosynthesis and related peroxidase isozyme changes in Catharanthus roseus. J Radiat Res Appl Sci. 2020;13:808–814. doi: 10.1080/16878507.2020.1777658. [DOI] [Google Scholar]
- Salehin M, Li B, Tang M, Katz E, Song L, Ecker JR, Kliebenstein DJ, Estelle M. Auxin-sensitive Aux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat Commun. 2019;10(1):1–9. doi: 10.1038/s41467-019-12002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F, Colinas M, Pollier J, Van Moerkercke A, Bossche RV, De Clercq R, Goossens A. An engineered combinatorial module of transcription factors boosts production of monoterpenoid indole alkaloids in Catharanthus roseus. Metab Eng. 2018;48:150–162. doi: 10.1016/j.ymben.2018.05.016. [DOI] [PubMed] [Google Scholar]
- Sharma A, Verma P, Mathur A, Mathur AK. Genetic engineering approach using early vinca alkaloid biosynthesis genes led to increased tryptamine and terpenoid indole alkaloids biosynthesis in differentiating cultures of Catharanthus roseus. Protoplasma. 2018;255(1):425–435. doi: 10.1007/s00709-017-1151-7. [DOI] [PubMed] [Google Scholar]
- Sharma A, Verma P, Mathur A, Mathur AK. Overexpression of tryptophan decarboxylase and strictosidine synthase enhanced terpenoid indole alkaloid pathway activity and antineoplastic vinblastine biosynthesis in Catharanthus roseus. Protoplasma. 2018;255(5):1281–1294. doi: 10.1007/s00709-018-1233-1. [DOI] [PubMed] [Google Scholar]
- Sharma A, Amin D, Sankaranarayanan A, Arora R, Mathur AK. Present status of Catharanthus roseus monoterpenoid indole alkaloids engineering in homo-and hetero-logous systems. Biotechnol Lett. 2020;42(1):11–23. doi: 10.1007/s10529-019-02757-4. [DOI] [PubMed] [Google Scholar]
- Shoji T, Yuan L. ERF gene clusters: working together to regulate metabolism. Trends Plant Sci. 2021;26(1):23–32. doi: 10.1016/j.tplants.2020.07.015. [DOI] [PubMed] [Google Scholar]
- Sibéril Y, Benhamron S, Memelink J, Giglioli-Guivarc’h N, Thiersault M, Boisson B, Doireau P, Gantet P. Catharanthus roseus G-box binding factors 1 and 2 act as repressors of strictosidine synthase gene expression in cell cultures. Plant Mol Biol. 2001;45(4):477–488. doi: 10.1023/A:1010650906695. [DOI] [PubMed] [Google Scholar]
- Singh SK, Patra B, Paul P, Liu Y, Pattanaik S, Yuan L. Revisiting the ORCA gene cluster that regulates terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Sci. 2020;293:110408. doi: 10.1016/j.plantsci.2020.110408. [DOI] [PubMed] [Google Scholar]
- Singh SK, Patra B, Paul P, Liu Y, Pattanaik S, Yuan L. BHLH IRIDOID SYNTHESIS 3 is a member of a bHLH gene cluster regulating terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Dir. 2021;5(1):e00305. doi: 10.1002/pld3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Smart N, Misawa M, Kurz W, Tallevi S, DiCosmo FJPcr. Increased accumulation of indole alkaloids by some cell lines of Catharanthus roseus in response to addition of vanadyl sulphate. Plant Cell Rep. 1987;6(2):142–145. doi: 10.1007/BF00276673. [DOI] [PubMed] [Google Scholar]
- Srivastava N, Srivastava A. Influence of some heavy metals on growth, alkaloid content and composition in Catharanthus roseus L. Ind J Pharm Sci. 2010;72(6):775. doi: 10.4103/0250-474X.84592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani N, Nazarian-Firouzabadi F, Shafeinia A, Sadr AS, Shirali M. The expression of terpenoid indole alkaloid (TIAs) pathway genes in Catharanthus roseus in response to salicylic acid treatment. Mol Biol Rep. 2020;47(9):7009–7016. doi: 10.1007/s11033-020-05759-y. [DOI] [PubMed] [Google Scholar]
- St-Pierre B, Laflamme P, Alarco AM, Luca E. The terminal O‐acetyltransferase involved in vindoline biosynthesis defines a new class of proteins responsible for coenzyme A‐dependent acyl transfer. Plant J. 1998;14(6):703–713. doi: 10.1046/j.1365-313x.1998.00174.x. [DOI] [PubMed] [Google Scholar]
- Stavrinides A, Tatsis EC, Caputi L, Foureau E, Stevenson CE, Lawson DM, Courdavault V, O’connor SE Structural investigation of heteroyohimbine alkaloid synthesis reveals active site elements that control stereoselectivity. Nat Commun. 2016;7(1):1–14. doi: 10.1038/ncomms12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, Singh SK, Patra B, Schluttenhofer C, Guo W, Pattanaik S, Yuan L. Cross-family transcription factor interaction between MYC2 and GBFs modulates terpenoid indole alkaloid biosynthesis. J Exp Bot. 2018;69(18):4267–4281. doi: 10.1093/jxb/ery229. [DOI] [PubMed] [Google Scholar]
- Sung YC, Lin CP, Chen JC. Optimization of virus-induced gene silencing in Catharanthus roseus. Plant Pathol. 2014;63(5):1159–1167. doi: 10.1111/ppa.12186. [DOI] [Google Scholar]
- Suttipanta N, Pattanaik S, Kulshrestha M, Patra B, Singh SK, Yuan L. The transcription factor CrWRKY1 positively regulates the terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 2011;157(4):2081–2093. doi: 10.1104/pp.111.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K, Pan Q. Catharanthus roseus. Cham: Springer; 2017. Strategies for enhancing alkaloids yield in Catharanthus roseus via metabolic engineering approaches; pp. 1–16. [Google Scholar]
- Tang Z, Rao L, Peng G, Zhou M, Shi G, Liang YJJoMPR. Effects of endophytic fungus and its elicitors on cell status and alkaloid synthesis in cell suspension cultures of Catharanthus roseus. J Med Plant Res. 2011;5(11):2192–2200. [Google Scholar]
- Thamm AM, Qu Y, De Luca V. Discovery and metabolic engineering of iridoid/secoiridoid and monoterpenoid indole alkaloid biosynthesis. Phytochem Rev. 2016;15:339–361. doi: 10.1007/s11101-016-9468-y. [DOI] [Google Scholar]
- Tonk D, Mujib A, Maqsood M, Ali M, Zafar NJPC, Tissue, Culture O. Aspergillus flavus fungus elicitation improves vincristine and vinblastine yield by augmenting callus biomass growth in Catharanthus roseus. Plant Cell Tiss Organ Cult. 2016;126(2):291–303. doi: 10.1007/s11240-016-0998-1. [DOI] [Google Scholar]
- Tossi VE, Regalado JJ, Iannicelli J, Laino LE, Burrieza HP, Escandon AS, Pitta-Alvarez SI. Beyond Arabidopsis: differential UV-B response mediated by UVR8 in diverse species. Front Plant Sci. 2019;10:780. doi: 10.3389/fpls.2019.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A, Manghwar H, Shaban M, Khan AH, Akbar A, Ali U, Ali E, Fahad S. Phytohormones enhanced drought tolerance in plants: a coping strategy. Environ Sci Pollut Res Int. 2018;25(33):33103–33118. doi: 10.1007/s11356-018-3364-5. [DOI] [PubMed] [Google Scholar]
- van der Fits L, Memelink J. ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science. 2000;289(5477):295–297. doi: 10.1126/science.289.5477.295. [DOI] [PubMed] [Google Scholar]
- van Der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R. The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem. 2004;11(5):607–628. doi: 10.2174/0929867043455846. [DOI] [PubMed] [Google Scholar]
- Van Moerkercke A, Steensma P, Schweizer F, Pollier J, Gariboldi I, Payne R, Bossche RV, Miettinen K, Espoz J, Purnama PC. The bHLH transcription factor BIS1 controls the iridoid branch of the monoterpenoid indole alkaloid pathway in Catharanthus roseus. Proc Natl Acad Sci. 2015;112(26):8130–8135. doi: 10.1073/pnas.1504951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Moerkercke A, Steensma P, Gariboldi I, Espoz J, Purnama PC, Schweizer F, Miettinen K, Vanden Bossche R, De Clercq R, Memelink J. The basic helix-loop‐helix transcription factor BIS 2 is essential for monoterpenoid indole alkaloid production in the medicinal plant Catharanthus roseus. Plant J. 2016;88(1):3–12. doi: 10.1111/tpj.13230. [DOI] [PubMed] [Google Scholar]
- Wang C-T, Liu H, Gao X-S, Zhang H-X. Overexpression of G10H and ORCA3 in the hairy roots of Catharanthus roseus improves catharanthine production. Plant Cell Rep. 2010;29(8):887–894. doi: 10.1007/s00299-010-0874-0. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xing S, Pan Q, Yuan F, Zhao J, Tian Y, Chen Y, Wang G, Tang K. Development of efficient Catharanthus roseus regeneration and transformation system using Agrobacterium tumefaciens and hypocotyls as explants. BMC Biotechnol. 2012;12(1):1–12. doi: 10.1186/1472-6750-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Li J, Hu L, Zhang T, Zhang G, Lou Y. OsMPK3 positively regulates the JA signaling pathway and plant resistance to a chewing herbivore in rice. Plant Cell Rep. 2013;32(7):1075–1084. doi: 10.1007/s00299-013-1389-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang Y, Yang J, Ma C, Zhang Y, Ge T, Qi Z, Kang Y. Arabidopsis ROOT HAIR DEFECTIVE3 is involved in nitrogen starvation-induced anthocyanin accumulation. J Integr Plant Biol. 2015;57(8):708–721. doi: 10.1111/jipb.12320. [DOI] [PubMed] [Google Scholar]
- Wang X, Pan Y-J, Chang B-W, Hu Y-B, Guo X-R, Tang Z-H. Ethylene-induced vinblastine accumulation is related to activated expression of downstream TIA pathway genes in Catharanthus roseus. BioMed Res Int. 2016 doi: 10.1155/2016/3708187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J, Goklany S, Rizvi N, Cram EJ, Lee-Parsons CW. Optimizing the transient fast agro-mediated seedling transformation (FAST) method in Catharanthus roseus seedlings. Plant Cell Rep. 2014;33(1):89–97. doi: 10.1007/s00299-013-1514-2. [DOI] [PubMed] [Google Scholar]
- Xiang L, Jian D, Zhang F, Yang C, Bai G, Lan X, Chen M, Tang K, Liao Z. The cold-induced transcription factor bHLH112 promotes artemisinin biosynthesis indirectly via ERF1 in Artemisia annua. J Exp Bot. 2019;70(18):4835–4848. doi: 10.1093/jxb/erz220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XB, Li S, Zhang RF, Zhao J, Chen YC, Zhao Q, Yao YX, You CX, Zhang XS, Hao YJ. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012;35(11):1884–1897. doi: 10.1111/j.1365-3040.2012.02523.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Grzech D, Koudounas K, Stander EA, Caputi L, Mimura T, Courdavault V, O’Connor SE. Improved virus-induced gene silencing allows discovery of a serpentine synthase gene in Catharanthus roseus. Plant Physiol. 2021 doi: 10.1093/plphys/kiab285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wen K-S, Ruan X, Zhao Y-X, Wei F, Wang Q. Response of plant secondary metabolites to environmental factors. Molecules. 2018;23(4):762. doi: 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, De Luca V. ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus. Proc Natl Acad Sci USA. 2013;110(39):15830–15835. doi: 10.1073/pnas.1307504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi SM, Karimi M, Venditti A. Plants adapted to arid areas: specialized metabolites. Nat Prod Res. 2019 doi: 10.1080/14786419.2019.1689500. [DOI] [PubMed] [Google Scholar]
- Zandalinas SI, Fritschi FB, Mittler R. Signal transduction networks during stress combination. J Exp Bot. 2020;71(5):1734–1741. doi: 10.1093/jxb/erz486. [DOI] [PubMed] [Google Scholar]
- Zárate R, Verpoorte R. Strategies for the genetic modification of the medicinal plant Catharanthus roseus (L.) G. Don. Phytochem Rev. 2007;6(2–3):475–491. doi: 10.1007/s11101-006-9020-6. [DOI] [Google Scholar]
- Zhang WJ, Björn LO. The effect of ultraviolet radiation on the accumulation of medicinal compounds in plants. Fitoterapia. 2009;80(4):207–218. doi: 10.1016/j.fitote.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu Y. Activation of salicylic acid–induced protein kinase, a mitogen-activated protein kinase, induces multiple defense responses in tobacco. Plant Cell. 2001;13(8):1877–1889. doi: 10.1105/TPC.010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hedhili S, Montiel G, Zhang Y, Chatel G, Pré M, Gantet P, Memelink J. The basic helix-loop‐helix transcription factor CrMYC2 controls the jasmonate‐responsive expression of the ORCA genes that regulate alkaloid biosynthesis in Catharanthus roseus. Plant J. 2011;67(1):61–71. doi: 10.1111/j.1365-313X.2011.04575.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu Z, Liu J, Lin S, Wang J, Lin W, Xu W. GA-DELLA pathway is involved in regulation of nitrogen deficiency-induced anthocyanin accumulation. Plant Cell Rep. 2017;36(4):557–569. doi: 10.1007/s00299-017-2102-7. [DOI] [PubMed] [Google Scholar]
- Zhang F, Xiang L, Yu Q, Zhang H, Zhang T, Zeng J, Geng C, Li L, Fu X, Shen Q. ARTEMISININ BIOSYNTHESIS PROMOTING KINASE 1 positively regulates artemisinin biosynthesis through phosphorylating AabZIP1. J Exp Bot. 2018;69(5):1109–1123. doi: 10.1093/jxb/erx444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Gou Y, Bai F, Bai G, Chen M, Zhang F, Liao Z. AaPP2C1 negatively regulates the expression of genes involved in artemisinin biosynthesis through dephosphorylating AaAPK1. FEBS Lett. 2019;593(7):743–750. doi: 10.1002/1873-3468.13350. [DOI] [PubMed] [Google Scholar]
- Zhao J, Verpoorte R. Manipulating indole alkaloid production by Catharanthus roseus cell cultures in bioreactors: from biochemical processing to metabolic engineering. Phytochem Rev. 2007;6(2):435–457. doi: 10.1007/s11101-006-9050-0. [DOI] [Google Scholar]
- Zheng Z, Wu M. Cadmium treatment enhances the production of alkaloid secondary metabolites in Catharanthus roseus. Plant Sci. 2004;166(2):507–514. doi: 10.1016/j.plantsci.2003.10.022. [DOI] [Google Scholar]
- Zhong Z, Liu S, Zhu W, Ou Y, Yamaguchi H, Hitachi K, Tsuchida K, Tian J, Komatsu S. Phosphoproteomics reveals the biosynthesis of secondary metabolites in Catharanthus roseus under ultraviolet-B radiation. J Proteome Res. 2019;18(9):3328–3341. doi: 10.1021/acs.jproteome.9b00267. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Liu S, Han S, Li Y, Tao M, Liu M, He Q, Chen S, Dufresne C, Wei Z, Tian J. Integrative omic analysis reveals the improvement of alkaloid accumulation by ultraviolet-B radiation and its upstream regulation in Catharanthus roseus. Ind Crops Prod. 2021;166:113448. doi: 10.1016/j.indcrop.2021.113448. [DOI] [Google Scholar]
- Zhu W, Yang B, Komatsu S, Lu X, Li X, Tian J. Binary stress induces an increase in indole alkaloid biosynthesis in Catharanthus roseus. Front Plant Sci. 2015;6:582. doi: 10.3389/fpls.2015.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]