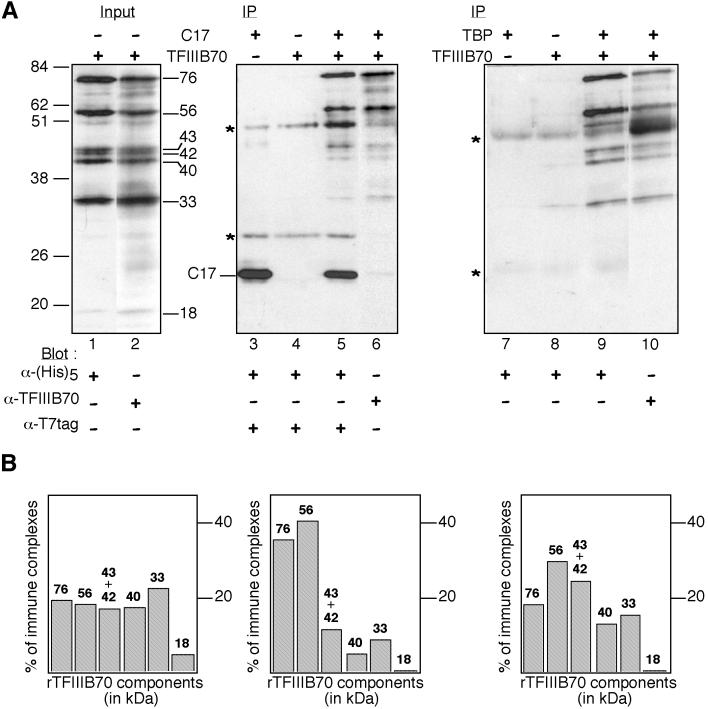
FIG. 5.
Coimmunoprecipitation assay of C17 and N-terminally truncated TFIIIB70 fragments. Crude extracts (60 μg) with (+) or without (−) T7-tagged C17 or His6-tagged rTFIIIB70 were preincubated for 45 min at 25°C and then mixed with magnetic beads coated with anti-T7 antibodies. Crude extracts (60 μg) with or without rTFIIIB70 were also preincubated with purified recombinant TBP (240 ng), as indicated, and then mixed with magnetic beads coated with anti-TBP antibodies. The beads were washed, and bound proteins were eluted by boiling the beads in loading buffer. (A) Western blot analysis. The input and bound proteins were analyzed by Western blotting using anti-T7, antipentahistidine, or anti-TFIIIB70 antibodies, as indicated. Asterisks indicate positions of immunoglobulin heavy and light chains; positions and molecular masses (in kilodaltons) of marker bands are indicated at the left. The molecular masses (in kilodaltons) of full-length and truncated rTFIIIB70 polypeptides present in crude extracts are shown. The position of rC17, migrating at 23 kDa, is indicated. IP, immunoprecipitation. (B) Quantification of rTFIIIB70 immune complexes. The immune complexes shown in panel A revealed with anti-TFIIIB70 antibodies (lanes 2, 6, and 10) were quantified with ImageQuant software. The results for each component of rTFIIIB70 are shown as percentage of total immune complexes.