Abstract
BACKGROUND:
The role of prenatal vitamin D sufficiency and supplementation in the development of childhood aeroallergen sensitization and allergic rhinitis remains uncertain.
OBJECTIVE:
To describe the association of prenatal vitamin D sufficiency with childhood allergic outcomes in participants of the Vitamin D Antenatal Asthma Reduction Trial, a randomized controlled trial of prenatal vitamin D supplementation.
METHODS:
We included 414 mother–offspring pairs with offspring aeroallergen sensitization data available at age 6 years in this analysis. We examined the association between prenatal vitamin D sufficiency status, based on vitamin D levels measured in the first and third trimesters, or vitamin D supplementation treatment assignment with the outcomes of aeroallergen sensitization, parent-reported clinical allergic rhinitis, parent-reported clinical allergic rhinitis with aeroallergen sensitization, food sensitization, any sensitization, eczema, and total IgE at ages 3 and 6 years.
RESULTS:
Compared with early and late insufficiency, early prenatal vitamin D insufficiency with late sufficiency was associated with reduced development of clinical allergic rhinitis with aeroallergen sensitization at 3 years (adjusted odds ratio [aOR] = 0.34; 95% confidence interval [CI], 0.13–0.82;P = .02) and 6 years (aOR = 0.54; 95% CI, 0.29–0.98; P = .05). At 6 years, clinical allergic rhinitis with sensitization was significantly decreased in offspring whose mothers received high-dose vitamin D (aOR= 0.54; 95% CI, 0.32–0.91; P = .02) compared with offspring whose mothers who received low-dose vitamin D. Associations of prenatal vitamin D with aeroallergen sensitization were strengthened among children who also developed asthma or who had a maternal history of atopy.
CONCLUSIONS:
Among mothers with first-trimester vitamin D insufficiency, we detected a protective effect of third-trimester prenatal vitamin D sufficiency on the development of clinical allergic rhinitis with aeroallergen sensitization at ages 3 and 6 years.
Keywords: Allergic rhinitis, Aeroallergen sensitization, Vitamin D, Prenatal
INTRODUCTION
Vitamin D has known immunomodulatory effects that may be important in the development of future allergic disease starting as early as the first trimester of fetal development.1,2 Human studies have shown that maternal vitamin D crosses the placenta freely and levels can be modified by maternal diet and supplementation. The increasing prevalence of vitamin D insufficiency worldwide has been suspected to be a factor in the increasing rates of allergic rhinitis and environmental aeroallergen sensitization.3–5 However, the role of prenatal vitamin D supplementation for the prevention of allergic rhinitis remains unclear, although several studies have suggested a protective effect of prenatal vitamin D sufficiency in allergic rhinitis and asthma in offspring.6–8
Achieving an understanding of the association between prenatal vitamin D insufficiency and the development of allergic rhinitis would have a marked impact on clinical recommendations to decrease this modifiable risk factor, especially in those with a genetic predisposition for atopy.9 Allergic rhinitis is a highly prevalent disease with a reported prevalence of up to 25% in children and over 40% in adults.10 Although allergic rhinitis alone is not life-threatening, its symptoms can have a significant impact on the quality of life as well as daily work and school performance. Furthermore, allergic rhinitis is a risk factor for asthma and can lead to poor asthma control and asthma morbidity.10
In this study, we hypothesize that maternal prenatal vitamin D sufficiency is associated with a risk reduction of offspring development of aeroallergen sensitization and allergic rhinitis in the Vitamin D Antenatal Asthma Reduction Trial (VDAART) birth cohort at high risk for atopy. In addition to examining the effect of prenatal vitamin D supplementation, we analyzed prenatal vitamin D levels in the first and third trimesters to capture a representation of vitamin D sufficiency status in early and late pregnancy. Our primary outcome of interest was aeroallergen sensitization at age 6 years. We examined secondary outcomes at age 3 and 6 years, including parent-reported clinical allergic rhinitis, clinical allergic rhinitis with aeroallergen sensitization, food allergen sensitization, any allergen sensitization, total IgE, and clinical eczema.
METHODS
Study design and participants
We performed a secondary analysis of the VDAART study, which is a randomized, double-blind placebo-controlled trial of 881 pregnant women randomized to 4400 IU vitamin D daily or placebo multivitamin with 400 IU vitamin D daily. Maternal–offspring pairs with available clinical data from offspring at age 3 and 6 years were used as the source for this analysis. Pregnant women who participated in VDAART were aged 18 to 39 years at 10 to 18 weeks of gestation and nonsmoking, with a personal history of asthma or atopy or a partner’s (biologic father of child’s) history of asthma or atopy, and intent to participate for at least 4 years. Mothers taking vitamin D supplements 2,000 IU/d or greater of vitamin D3 and those enrolled in VDAART for a prior pregnancy were excluded from participation. A full description of VDAART study design, inclusion, and exclusion criteria was previously given by Litonjua et al.11,12 The primary outcomes of interest in VDAART were doctor-diagnosed asthma and recurrent wheeze. Secondary outcomes included allergic sensitization, doctor’s diagnosis of eczema, and allergic rhinitis. Blood samples were prenatally collected from mothers at 10 to 18 weeks of gestation and 32 to 38 weeks of gestation, and their offspring at age 3 and 6 years. Written consent was obtained from mothers; the study was approved by the institutional review boards at the three clinical centers (Boston, St Louis, and San Diego) and Brigham and Women’s Hospital.
Prenatal vitamin D measurement and sufficiency status definition
Maternal vitamin D was measured at two prenatal time points: early pregnancy at 10 to 18 weeks of gestation and late pregnancy at 32 to 38 weeks of gestation. In this study, vitamin D levels refer to the measurement of total 25-hydroxyvitamin D, which was obtained according to the VDAART protocol.11 The DiaSorin LIAISON (Saluggia, Italy) chemiluminescence immunoassay was performed on maternal serum samples at the Channing Division of Network Medicine, Brigham and Women’s Hospital.13
Vitamin D sufficiency was defined according to the Endocrine Society’s recommendation for maximum bone health benefits as a 25-hydroxyvitamin D level of 30 ng/mL or greater.14 Vitamin D sufficiency status at early and late pregnancy was the main exposure of interest in this study. Prenatal vitamin D treatment assignment was also examined in relation to the defined allergic outcomes.
Clinical outcomes in offspring
Aeroallergen sensitization at age 6 years was defined by a positive serum-specific IgE of 0.35 kU/L or greater to one or more of seven measured aeroallergens. Environmental aeroallergens assessed included Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat dander, dog dander, grass mix, German cockroach, and Alternaria alternata. Tree pollen sensitization was included at age 3 years but was unavailable at 6 years. Serum-specific IgE assays were performed using the UniCAP system (Phadia AB, Portage, Mich). Of the initial 806 maternal–offspring pairs with clinical data available after birth, 414 had sensitization data at age 6 years. Any offspring with incomplete aeroallergen sensitization data and negative serum-specific IgE values in the available data at age 3 or 6 years were excluded from the analysis because we could not confirm these subjects’ aeroallergen sensitization status (n = 10 for age 3 years and n = 0 for age 6 years).
We examined other outcomes at age 3 and 6 years, including parent-reported clinical allergic rhinitis, food sensitization, any food and/or aeroallergen sensitization, clinical eczema, and serum total IgE levels. Clinical allergic rhinitis was based on parent survey responses to the question “Has a health care provider said that the child has hay fever, allergic rhinitis, or allergies involving the eyes or nose?” To increase specificity for true allergic rhinitis, we defined a composite outcome of parent-reported clinical allergic rhinitis with positive aeroallergen sensitization; subjects with both conditions were compared with those with only aeroallergen sensitization, only allergic rhinitis, and neither aeroallergen sensitization nor allergic rhinitis. Food sensitization was defined as positive if a subject had at least one serum-specific IgE value of 0.35 kU/L or greater to peanut, soybean, egg white, milk, or walnut. Any sensitization was defined as having at least one positive serum-specific IgE to one of the 13 measured food and environmental allergens. Clinical eczema was determined by parental report of physician’s diagnosis of eczema.
Statistical analysis
All statistical analyses were performed as two-sided tests with a prespecified significance level of α = 0.05, using the R statistical package (version 3.6.3, R Core Team, Vienna Austria). Based on prior knowledge, study site, maternal education, child sex, and child race and ethnicity were included in the models as potential confounders. We also adjusted for baseline characteristics of offspring that were associated with aeroallergen sensitization using Student t test or chi-square test (P < .05) (Table I), which included preterm birth, parental asthma, and child body mass index (BMI) at age 3 or 6 years.
TABLE I.
Baseline characteristics in study population by primary outcome at age 6 ya
| Subject characteristic | Aeroallergen sensitization (n = 168) | Nonsensitized (n = 246) | P |
|---|---|---|---|
| Maternal age, y (mean [SD]) | 27.0 (5.2) | 27.6 (5.6) | .27 |
| Maternal education: less than college graduation | 123 (73.2) | 151 (61.4) | .02b |
| Treatment arm: high-dose prenatal vitamin D (4,400 IU/d) | 79 (47.0) | 134 (54.5) | .17 |
| Study site | .38 | ||
| Boston | 46 (27.4) | 74 (30.1) | |
| San Diego | 54 (32.1) | 89 (36.2) | |
| St Louis | 68 (40.5) | 83 (33.7) | |
| Female sex | 65 (38.7) | 125 (50.8) | .02b |
| Child race/ethnicity | .41 | ||
| Black | 72 (42.9) | 97 (39.4) | |
| Hispanic/other | 71 (42.3) | 100 (40.7) | |
| White | 25 (14.9) | 49 (19.9) | |
| Child body mass index at age 6 y (mean, SD) | 17.30 (3.29) | 16.57 (2.43) | .02b |
| Preterm birth | 9 (5.4) | 29 (11.8) | .04b |
| Birth mode: vaginal | 113 (67.3) | 177 (72.0) | .36 |
| Perinatal antibiotics | 65 (38.7) | 105 (42.7) | .48 |
| Breastfeeding ≥4 mo | 64 (38.1) | 105 (42.7) | .10 |
| Season of birth | .47 | ||
| Fall | 50 (29.8) | 68 (27.6) | |
| Spring | 41 (24.4) | 52 (21.1) | |
| Summer | 45 (26.8) | 63 (25.6) | |
| Winter | 32 (19.0) | 63 (25.6) | |
| Older siblings: yes | 103 (61.3) | 132 (53.7) | .15 |
| Daycare by age 6 y | 110 (65.5) | 150 (61.0) | .98 |
| Cat in home | 15 (8.9) | 37 (15.0) | .08 |
| Dog in home | 37 (22.0) | 60 (24.4) | .40 |
| Parental asthma | 107 (63.7) | 131 (53.3) | .05b |
| Parental hay fever | 131 (78.0) | 189 (76.8) | .88 |
| Parental eczema | 63 (37.5) | 115 (46.7) | .08 |
| Asthma by age 6 y | 56 (33.3) | 46 (18.7) | .001b |
Missingness includes breastfeeding for 4 mo (n = 28), cat (n = 8), dog (n = 10), and daycare status (n = 28).
Data are given as n (%) of individuals unless otherwise specified.
Indicates statistical significance (P < .05).
Using logistic regression, we tested associations between binary outcome variables and prenatal vitamin D sufficiency and treatment assignment. We tested associations between total IgE, which was log-transformed before analysis, and prenatal vitamin D in linear regression models. Based on a threshold of 30 ng/mL or greater, four prenatal exposure levels were designated using the two vitamin D collection time points (10–18 and 32–38 weeks of gestation): early and late insufficiency, early sufficiency and late insufficiency, early insufficiency and late sufficiency, and early and late sufficiency. Because of the small sample size (n = 21 and 14 among mothers of offspring with outcome data available at age 3 and 6 years, respectively), subjects in the early sufficient/late insufficient category were excluded from the analysis. Prenatal vitamin D sufficiency was also analyzed as a numeric variable to generate P values for tests of trend (1 for insufficient status in early and late pregnancy, 2 for early insufficient/late sufficient, and 3 for sufficient in early and late pregnancy). To determine the contribution of treatment assignment to the association between clinical outcomes and prenatal vitamin D sufficiency, we used the Karlson-Holm-Breen method to compare nested regression models.15 To evaluate for effect modification, we repeated key analyses stratified by maternal atopy status with atopy defined by reported history of asthma, allergic rhinitis, and/or eczema. We also evaluated the interaction between prenatal vitamin D sufficiency and child clinical asthma status on allergic outcomes of interest, because we hypothesized that offspring with asthma and allergic sensitization could represent a more severe atopic phenotype or distinct endotype with a relationship to prenatal vitamin D that could differ from that seen in children with atopy but no asthma. Thus, we also repeated key analyses stratified by child clinical asthma status. For all of these models, we obtained crude and adjusted odds ratios along with confidence intervals (CIs) and P values.
RESULTS
Participant baseline characteristics
The original VDAART study base was composed of 806 mother–offspring pairs who participated in the prenatal vitamin D supplementation trial. For this secondary analysis, offspring with aeroallergen sensitization data at age 3 or 6 years were included in the analysis. All VDAART participants were asked to provide a blood sample at age 3 and 6 years; a subset of participants agreed to this. Baseline characteristics of those with available aeroallergen sensitization data from age 6 years (n = 414) were comparable to characteristics of maternal–offspring pairs who were excluded from the analysis owing to missing aeroallergen sensitization data (n = 405) (see Table E1 in this article’s Online Repository at www.jaci-inpractice.org). We excluded subjects with incomplete vitamin D data during pregnancy (n = 25 and 14 among mothers of offspring with outcome data available at age 3 and 6 years, respectively) and those in the early sufficient/late insufficient prenatal vitamin D category owing to small sample size (n = 21 and 14 among mothers of offspring with outcome data available at age 3 and 6 years, respectively) (Figure 1).
FIGURE 1.
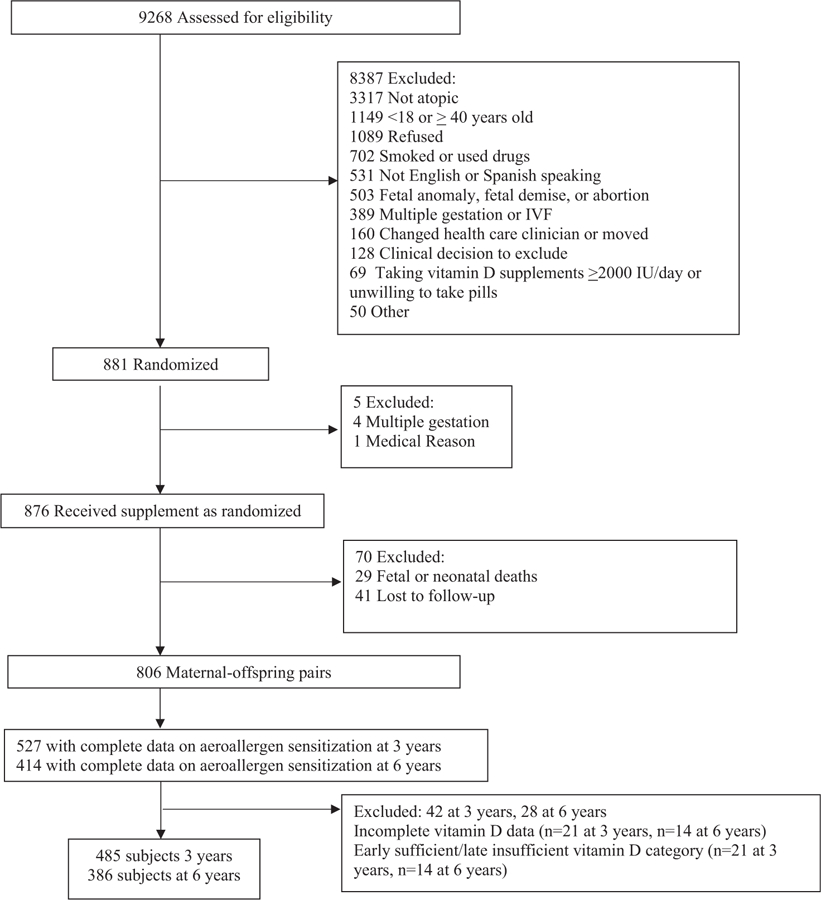
Consolidated Standards of Reporting Trials diagram. IVF, in vitro fertilization.
Of 414 offspring, 168 with available sensitization data had aeroallergen sensitization at age 6 years (40.5%). Most of those (n = 142; 85%) had polysensitization to more than one aeroallergen, whereas the remainder were monosensitized to only one aeroallergen (n = 26; 15%). Of the 168 subjects with aeroallergen sensitization at age 6 years, 78 also had parent-reported clinical allergic rhinitis and therefore met criteria for the composite outcome of allergic rhinitis with aeroallergen sensitization. Thirty-six subjects had allergic rhinitis with aeroallergen sensitization at age 3 years.
In comparisons of baseline characteristics, offspring with aeroallergen sensitization at age 6 years and nonsensitized subjects significantly differed on maternal education, child sex, child BMI, preterm birth status, and parental asthma (Table I). Those with aeroallergen sensitization were more likely than nonsensitized subjects to have mothers who were not college graduates (73.2% vs 61.4%; P = .02), have a parent with asthma (63.7% vs 53.3%; P =.05), and have a higher BMI at age 6 years (mean, 17.30 vs 16.57; P = .02). Those with aeroallergen sensitization were less likely to be female (38.7% vs 50.8%; P = .02) or to have been born before 37 weeks of gestation (5.4% vs 11.8%; P = .04). These potential confounders were included as covariates in subsequent analyses, in addition to covariates selected a priori, including study site and child race or ethnicity. Baseline characteristics were otherwise balanced between offspring with aeroallergen sensitization and nonsensitized subjects (Table I). Offspring with and without aeroallergen sensitization at the earlier age 3 years point were largely comparable on baseline characteristics, with the exception of a higher frequency of parental asthma in those with aeroallergen sensitization (67% among aeroallergen sensitization vs 54% among nonsensitized subjects) (see Table E2 in this article’s Online Repository at www.jaci-inpractice.org).
Association of prenatal vitamin D sufficiency with aeroallergen sensitization and allergic rhinitis
In both crude and adjusted analyses at age 3 and 6 years, there were no significant associations between prenatal vitamin D sufficiency status and aeroallergen sensitization (Figure 2 and Table II; see Table E3 in this article’s Online Repository at www.jaci-inpractice.org). However, upon examining associations of prenatal vitamin D sufficiency status with the composite outcome of clinical allergic rhinitis with aeroallergen sensitization at age 6 years, we observed reduced allergic rhinitis with sensitization among offspring of mothers with early prenatal vitamin D insufficiency and late vitamin D sufficiency compared with those with vitamin D insufficiency in early and late pregnancy (odds ratio = 0.47; 95% CI, 0.26–0.84; P = .01). This association was preserved in models adjusted for potential confounders (adjusted odds ratio [aOR] = 0.54; 95% CI, 0.29–0.98; P = .05) (Figure 2). At age 3 years, the same inverse association of early vitamin D insufficiency with late vitamin D sufficiency and clinical allergic rhinitis with aeroallergen sensitization, compared with vitamin D insufficiency in early and late pregnancy, was observed (aOR = 0.34; 95% CI, 0.13–0.82; P = .02) (Table E3). In contrast, we found no association between vitamin D sufficiency in both early and late pregnancy, compared with vitamin D insufficiency in early and late pregnancy and offspring allergic rhinitis with sensitization (Figure 2 and Tables II and E3.
FIGURE 2.
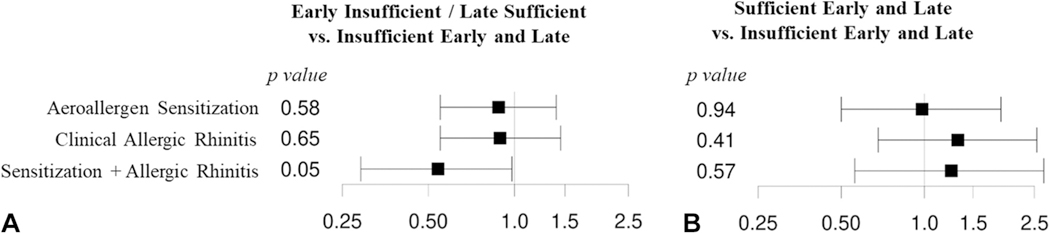
Forest plots depicting odds ratio and 95% confidence intervals of associations of prenatal vitamin D and allergic outcomes at age 6 years obtained from logistic regression models adjusted for child sex, preterm birth, child race/ethnicity, child body mass index at age 6 years, maternal education, parental asthma, and study site. (A) Comparison of vitamin D insufficiency early in pregnancy/vitamin D sufficiency late in pregnancy (n = 157 subjects; 60 with aeroallergen sensitization, 54 with clinical allergic rhinitis, and 20 with sensitization and allergic rhinitis) with vitamin D insufficiency early and late in pregnancy (n = 165 subjects; 71 with aeroallergen sensitization, 69 with clinical allergic rhinitis, and 39 with sensitization and allergic rhinitis). (B) Comparison of vitamin D sufficiency early and late in pregnancy (n = 64 subjects; 24 with aeroallergen sensitization, 25 with clinical allergic rhinitis, and 14 with sensitization and allergic rhinitis) with vitamin D insufficiency early and late in pregnancy.
TABLE II.
Allergic outcomes at age 6 ya
| Univariable |
Multivariablea |
|||||
|---|---|---|---|---|---|---|
| Subject characteristic | Odds ratio (95% CI) | P | P for trend | Adjusted odds ratio (95% CI) | P | P for trend |
| Aeroallergen sensitization | ||||||
| Insufficient in early and late prenatalb (n = 165) | Reference | — | .36 | Reference | — | .80 |
| Early insufficient/late sufficient (n = 157) | 0.82 (0.52–1.28) | .38 | 0.88 (0.55–1.41) | .58 | ||
| Sufficient in early and late prenatal (n = 64) | 0.79 (0.44–1.43) | .45 | 0.98 (0.5–1.89) | .94 | ||
| Clinical allergic rhinitis | ||||||
| Insufficient in early and late prenatal (n = 165) | Reference | — | .45 | Reference | — | .60 |
| Early insufficient/late sufficient (n = 157) | 0.73 (0.46–1.14) | .17 | 0.89 (0.55–1.45) | .65 | ||
| Sufficient in early and late prenatal (n = 64) | 0.89 (0.49–1.6) | .70 | 1.32 (0.68–2.55) | .41 | ||
| Aeroallergen sensitization and clinical allergic rhinitis | ||||||
| Insufficient in early and late prenatal (n = 165) | Reference | — | .29 | Reference | — | .78 |
| Early insufficient/late sufficient (n = 157) | 0.47 (0.26–0.84) | .01c | 0.54 (0.29–0.98) | .05c | ||
| Sufficient in early and late prenatal (n = 64) | 0.9 (0.44–1.78) | .78 | 1.25 (0.56–2.70) | .57 | ||
| Food sensitization | ||||||
| Insufficient in early and late prenatal (n = 165) | Reference | — | .15 | Reference | — | 0.55 |
| Early insufficient/late sufficient (n = 157) | 0.81 (0.51–1.27) | .35 | 0.90 (0.56–1.45) | .67 | ||
| Sufficient in early and late prenatal (n = 64) | 0.65 (0.34–1.19) | .17 | 0.83 (0.41–1.63) | .59 | ||
| Any sensitization | ||||||
| Insufficient in early and late prenatal (n = 165) | Reference | — | Reference | — | ||
| Early insufficient/late sufficient (n = 157) | 0.84 (0.54–1.31) | .45 | .09 | 0.96 (0.60–1.53) | .90 | .54 |
| Sufficient in early and late prenatal (n = 64) | 0.59 (0.33–1.06) | .08 | 0.80 (0.41–1.54) | .50 | ||
| Clinical eczema | ||||||
| Insufficient in early and late prenatal (n = 165) | Reference | — | .08 | Reference | — | .90 |
| Early insufficient/late sufficient (n = 157) | 0.96 (0.62–1.5) | .87 | 1.22 (0.76–1.97) | .40 | ||
| Sufficient in early and late prenatal (n = 64) | 0.55 (0.31–0.98) | .05c | 0.94 (0.49–1.82) | .86 | ||
|
| ||||||
| Total IgE | β (95% CI) | P | P for trend | Adjusted β (95% CI) | P | P |
|
| ||||||
| Insufficient in early and late prenatal (n = 165) | Reference | — | .05c | Reference | — | .31 |
| Early insufficient/Late sufficient (n = 157) | −0.26 (–0.62 to 0.11) | .16 | −0.17 (−0.53 to 0.19) | .36 | ||
| Sufficient in early and late prenatal (n = 64) | −0.44 (−0.92 to 0.03) | .07 | −0.22 (−0.72 to 0.28) | .39 | ||
CI, confidence interval.
Adjusted for maternal education, study site, preterm birth, child sex, child race and ethnicity, parental asthma, and child body mass index at age 6 y.
In the following order: baseline prenatal vitamin D (10–18 wk gestational age), third trimester prenatal vitamin D (32–38 wk gestational age).
Indicates statistical significance (P < .05).
Most subjects in the early vitamin D insufficient and late vitamin D sufficient category were assigned to vitamin D supplementation in the VDAART study (82%), whereas a lower proportion of subjects in the early and late vitamin D–insufficient and early and late vitamin D–sufficient categories were assigned to high-dose vitamin D treatment (24% and 55%, respectively) (see Table E4 in this article’s Online Repository at www.jaci-inpractice.org). This observation prompted us to examine the relationship between vitamin D supplementation and allergic rhinitis with aeroallergen sensitization. The effect of high-dose versus low-dose prenatal vitamin D treatment assignment on childhood aeroallergen sensitization and allergic rhinitis at age 3 and 6 years in VDAART was previously reported.7,11 Among VDAART subjects analyzed in the current study, high-dose prenatal vitamin D supplementation appeared to be beneficial in reducing clinical allergic rhinitis with aeroallergen sensitization at age 6 years in both crude and adjusted analysis (odds ratio = 0.52; 95% CI, 0.31–0.86, P = .01; aOR = 0.54, 95% CI, 0.32–0.91, P = .02). At age 3 years, the effect of the vitamin D treatment group was suggestive of a protective effect of high-dose vitamin D on allergic rhinitis with sensitization, but the results did not reach statistical significance (aOR = 0.60; 95% CI, 0.29–1.2; P = .15). To estimate the extent to which treatment assignment accounted for associations between prenatal vitamin D sufficiency status and allergic rhinitis with sensitization, we used the Karlson-Holm-Breen method to compare logistic regression models with and without adjustment for treatment assignment. We found that an estimated 29.9% of the observed effect on allergic rhinitis with sensitization was attributed to VDAART treatment assignment when we compared subjects with early vitamin D insufficiency and late sufficiency with those with early and late vitamin D insufficiency.
We next performed analyses to evaluate maternal atopy and child asthma as potential effective modifiers of the association between prenatal vitamin D and allergic outcomes in offspring. The observation of decreased allergic rhinitis with sensitization among offspring of mothers with early prenatal vitamin D insufficiency and late vitamin D sufficiency compared with those with early and late vitamin D insufficiency was augmented among 360 of 414 subjects with a history of maternal atopy (aOR = 0.44; 95% CI, 0.22–0.83; P = .01). This suggested that among mothers with vitamin D insufficiency early in pregnancy, vitamin D sufficiency late in pregnancy has a stronger protective effect on offspring’s allergic rhinitis when a history of maternal atopy is also present.
We hypothesized that children with allergic sensitization who also have asthma may represent a more severe phenotype of atopy and a distinct atopy endotype, and thus the association of prenatal vitamin D with aeroallergen sensitization may differ between children with and without asthma. We found a significant interaction between childhood asthma and prenatal vitamin D status on the outcome of aeroallergen sensitization at age 6 years in a comparison of maternal early vitamin D insufficiency with late sufficiency versus vitamin D insufficiency in early and late pregnancy (P = .04). In stratified analyses, among children with asthma (n = 90), aeroallergen sensitization at age 6 years was significantly less frequent in offspring of mothers with vitamin D insufficiency early in pregnancy and sufficiency late in pregnancy (16 of 37; 43%) compared with those who had vitamin D insufficiency in early and late pregnancy (30 of 47; 64%; aOR = 0.35; 95% CI, 0.12–0.96; P = .05) (Figure 3). This association was not seen among children without asthma (n = 312; aOR = 1.31; 95% CI, 0.75–2.33; P = .35). No significant associations between prenatal vitamin D status and aeroallergen sensitization at age 3 years were detected within either asthma strata (see Table E5 in this article’s Online Repository at www.jaci-inpractice.org).
FIGURE 3.
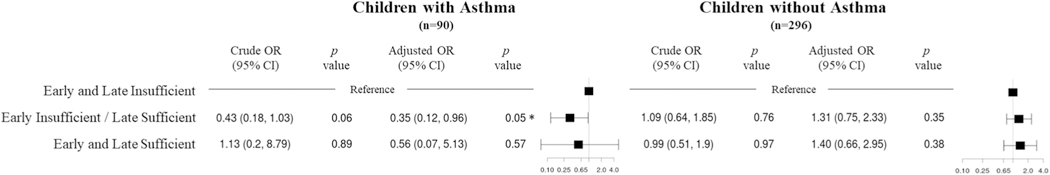
Association of vitamin D sufficiency status in pregnancy and aeroallergen sensitization at age 6 years stratified by children who received any asthma diagnosis between age 0 and 6 years and those with no asthma diagnosis between age 0 and 6 years. Forest plot shows the corresponding odds ratio (OR) and 95% confidence intervals (CIs) for each category of vitamin D sufficiency obtained from logistic regression models adjusted for child sex, preterm birth, child race/ethnicity, child body mass index at age 6 years, maternal education, parental asthma, and study site. The number of subjects with aeroallergen sensitization/total number of subjects in each vitamin D category is: early and late insufficient (30 of 47 with asthma, and 41 of 118 without asthma), early insufficient/late sufficient (16 of 37 with asthma, and 44 of 120 without asthma), and early and late sufficient (four of six with asthma, and 20 of 58 without asthma).
Association of prenatal vitamin D sufficiency with other outcomes
There were no significant associations in adjusted analyses between prenatal vitamin D and the secondary outcomes of food sensitization, any sensitization, and total IgE level at age 3 or 6 years (Tables II and E3). There were several significant associations in the crude analyses. Details of these analyses are available in the Supplementary Materials (in this article’s Online Repository at www.jaci-inpractice.org).
DISCUSSION
In this analysis of a diverse sample of maternal–offspring pairs, among mothers with vitamin D insufficiency in the first trimester, we detected a significant effect of vitamin D sufficiency in the third trimester on the composite outcome of aeroallergen sensitization and clinical allergic rhinitis. To our knowledge, this is the first study to report an association of prenatal vitamin D sufficiency status and high-dose supplementation with the composite outcome of those with both parent-reported allergic rhinitis and positive serum IgE to at least one aeroallergen. Prior studies examining the relationship between vitamin D and allergic rhinitis defined the outcome based on either survey-reported allergic rhinitis or aeroallergen sensitization data.6,16–18 The strength of this composite outcome is that it more likely represents those with true allergic rhinitis disease. The protective effect of late prenatal vitamin D sufficiency among mothers with early pregnancy vitamin D insufficiency was augmented among those with a history of maternal atopy. Furthermore, our study found that among children with asthma, who may exhibit a distinct and potentially more severe endotype of allergic sensitization, there was a protective effect of early pregnancy vitamin D insufficiency with late pregnancy vitamin D sufficiency on aeroallergen sensitization at age 6 years compared with vitamin D insufficiency in both early and late pregnancy. This is notable because comorbidity between asthma and rhinitis and the importance of allergic rhinitis control in decreasing asthma severity are well-established.10 This effect was not observed among those with childhood asthma at age 3 years, likely owing to the overall lower proportion of aeroallergen sensitization at age 3 years (148 of 531; 28%) compared with age 6 years (168 of 414; 40.5%), and is consistent with the knowledge that aeroallergen sensitization continues to develop throughout early childhood.19,20
Several baseline characteristics associated with allergic risk in the literature, including lower maternal education, male sex, higher BMI at age 6 years, preterm birth, and parental history of asthma, were also present in significantly higher proportions in the current study cohort among those with aeroallergen sensitization at age 6 years of age.16,17 Those with aeroallergen sensitization were also less likely to have a pet in the home and more likely to be born by cesarean section compared with those without aeroallergen sensitization, although these differences were not statistically significant. These observed differences in those with and without atopy highlight the complex, multifactorial nature of atopy.
An understanding of the role of vitamin D in regulation of the immune system and development of allergic disease has continued to expand with evidence of a broad range of immunologic effects including the induction of regulatory T cells, decreased B-cell IgE secretion, enhanced epithelial function, and in utero effects on lung development.1,18 Vitamin D has been shown to attenuate childhood asthma risk, although prenatal supplementation alone cannot entirely abolish the development of asthma.7,8,21,22 However, as a modifiable factor, it remains important to understand the role of vitamin D in prenatal and early life on allergic disease, to make appropriate clinical recommendations. Recent non-atopically enriched birth cohort studies on the effect of prenatal vitamin D exposure through diet and supplementation or measured serum sufficiency and development of allergic rhinitis revealed conflicting results ranging from null to protective or nonlinear effects of prenatal and perinatal vitamin D.6,23–25 A prior VDAART analysis also reported a null effect of high-dose compared with low-dose prenatal vitamin D supplementation on the outcomes of clinical allergic rhinitis and eczema at age 6 years but did not investigate these outcomes in relation to overall measured maternal prenatal vitamin D sufficiency.7 In this analysis, we were able to account for the differences in baseline vitamin D levels and treatment noncompliance by using serum vitamin D levels from the first and third trimesters rather than performing an intention-to-treat analysis of prenatal vitamin D supplementation. This study also contributes to the existing literature because it investigates the effect of prenatal vitamin D sufficiency on the development of allergic sensitization with associated clinical symptoms of allergic rhinitis in offspring of the VDAART cohort, which is enriched with an atopic family history and thus confers a greater risk for atopy to the offspring.26,27 The variable results of prior studies may be due to the difficulty of quantifying multiple sources of vitamin D exposure and longitudinal vitamin D sufficiency, as well as the overall high prevalence of either rhinitis symptoms or aeroallergen sensitization alone without true allergic rhinitis disease. We address both of these concerns in this study by characterizing longitudinal prenatal vitamin D sufficiency and examining the outcome of combined aeroallergen sensitization and allergic rhinitis.
Given the reduced risk for aeroallergen sensitization and allergic rhinitis that we observed in mothers who were vitamin D–insufficient early in pregnancy and vitamin D–sufficient in late pregnancy, we would have expected to see a similar effect in the group with vitamin D sufficiency in early and late pregnancy. We speculate that sample size limitation decreased the study’s power and hence our ability to detect a significant difference between subjects who were vitamin D sufficient in early and late the prenatal period and those who were vitamin D insufficient in early and late pregnancy. Another possibility is that the protective effect seen in the early-insufficient and late-sufficient prenatal vitamin D group resulted from the high proportion of subjects in this group who were randomized to high-dose prenatal vitamin D supplementation (129 of 157; 82%), in contrast to the group with vitamin D sufficiency in early and late pregnancy (35 of 64; 55%). This is supported by the finding that among mothers with vitamin D insufficiency early in pregnancy, approximately 30% of the association of later vitamin D sufficiency with reduced offspring allergic rhinitis with sensitization is caused by high-dose vitamin supplementation during pregnancy. This is also supported by the observed association of prenatal high-dose vitamin D supplementation with reduced allergic rhinitis with sensitization at age 6 years. It is possible that a spot serum measurement of vitamin D does not always accurately reflect longer-term active vitamin D and tissue stores to which subjects in the high-dose treatment arm would be exposed in early and late pregnancy, and so high-dose vitamin D supplementation may have protected against offspring allergic rhinitis beyond effects represented by circulating 25-hydroxyvitamin D.
There were several limitations to this study. There was a much smaller sample size in the early sufficient/late insufficient prenatal vitamin D exposure category than other prenatal vitamin D sufficiency categories, which limited our ability to detect and report significant differences in this group compared with those with vitamin D insufficiency in early and late pregnancy, although other studies showed that first-trimester vitamin D sufficiency may decrease atopic risk in childhood.1,28 We were also limited regarding the assessment of the child’s vitamin D status, so the results of this study can reflect only the importance of prenatal vitamin D, but thus it may be confounded by any postnatal vitamin D supplementation, which has also been shown to have an impact on allergic outcomes.29–32 We examined outcome measures at age 3 and 6 years, and a trend toward a protective effect of vitamin D on decreased total IgE and any aeroallergen or food sensitization was noted at 3 years, but not at 6 years. The differential effects on sensitization seen at age 3 and 6 years may be the result of a waning effect of prenatal vitamin D and increased effects of postnatal factors not analyzed here.
This study demonstrates a protective effect of third-trimester prenatal vitamin D sufficiency in mothers who had first-trimester vitamin D insufficiency on the development of parent-reported childhood allergic rhinitis with sensitization by age 6 years, particularly in attenuating the increased baseline risk among children who also developed asthma or who had a maternal history of atopy. This result is encouraging because it shows that prenatal vitamin D sufficiency appears to be beneficial even if not attained in the first trimester. In addition, in the subset of the VDAART birth cohort, prenatal high-dose vitamin D supplementation was associated with a decrease in aeroallergen sensitization at 6 years. Further studies in populations with high atopic risk are needed to elucidate the mechanisms of action and effect of prenatal and early-life vitamin D on the development of allergic disease.
Supplementary Material
What is already known about this topic?
Vitamin D has known immunomodulatory effects starting in utero. The role of prenatal vitamin D sufficiency in the development of childhood allergic rhinitis and aeroallergen sensitization is unclear.
What does this article add to our knowledge?
Prenatal vitamin D sufficiency in the third trimester and high-dose vitamin D supplementation may attenuate the risk for childhood allergic rhinitis and aeroallergen sensitization, especially among those with concurrent asthma or history of maternal atopy.
How does this study impact current management guidelines?
In populations with atopic risk, prenatal vitamin D supplementation may be beneficial in reducing offspring risk for allergic disease. Further studies on the prevention of allergic disease should consider the role of prenatal vitamin D.
Acknowledgments
The authors thank the study coordinators and participants of the VDAART for their contributions and support.
The National Health, Lung and Blood Institute (NHLBI) awarded Grants R01 HL091528 and UH3 OD023268 to S. T. Weiss and A. A. Litonjua. Y-C. S. Chen is supported by Grants T-32 HL007427 and T-32 AI007306 from the NHLBI. K. A. Lee-Sarwar is supported by Grant K08 from the NHLBI (K08HL148178), and H. Mirzakhani is supported by Grant K01 from the NHLBI (1K01HL146977–01A1).
Abbreviations used
- aOR
Adjusted odds ratio
- BMI
Body mass index
- CI
Confidence interval
- VDAART
Vitamin D Antenatal Asthma Reduction Trial
Footnotes
Conflicts of interest: A. A. Litonjua has received author royalties from UpToDate, Inc. S. T. Weiss has received royalties from UpToDate, Inc. L. B. Bacharier participates on the Data Safety Monitoring Board of DBV Technologies. A. Beigelman has received research support from the NHLBI and holds stock from DBV Technologies. R. S. Zeiger is a consultant for AstraZeneca, DBV Technologies, Genentech, Inc, GlaxoSmithKline, Novartis, and Regeneron, and has received research support from Aerocrine, AstraZeneca, Genentech, Inc, GlaxoSmithKline, MedImmune, Merck, the NHLBI, Quest Diagnostics, and TEVA Pharmaceuticals. G. T. O’Connor is a coinvestigator on a grant from Janssen Pharmaceuticals to Boston University that funds a study of the pathogenesis of chronic obstructive pulmonary disease. V. J. Carey has received research support from Bayer, Inc. H. Mirzakhani has received research support from the NHLBI. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Mirzakhani H, Al-Garawi A, Weiss ST, Litonjua AA. Vitamin D and the development of allergic disease: how important is it? Clin Exp Allergy 2015;45:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litonjua AA. The role of vitamin D in the development, exacerbation, and severity of asthma and allergic diseases. Respir Med 2012;3:201–38. [Google Scholar]
- 3.Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss MS, editors. White Book on Allergy: Executive Summary. Milwaukee, WI: World Allergy Organization; 2013. [Google Scholar]
- 4.Liu NQ, Kaplan AT, Lagishetty V, Ouyang YB, Ouyang Y, Simmons CF, et al. Vitamin D and the regulation of placental inflammation. J Immunol 2011;186:5968–74. [DOI] [PubMed] [Google Scholar]
- 5.Ginde AA, Liu MC, Camargo CA. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med 2009;169:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunyavanich S. Prenatal, perinatal, and childhood vitamin D exposure and their association with childhood allergic rhinitis and allergic sensitization. J Allergy Clin Immunol 2016;137:1063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litonjua AA, Carey VJ, Laranjo N, Stubbs BJ, Mirzakhani H, O’Connor GT, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med 2020;382:525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu M, Litonjua AA, O’Connor GT, Zeiger RS, Bacharier L, Schatz M, et al. Effect of early and late prenatal vitamin D and maternal asthma status on offspring asthma or recurrent wheeze. J Allergy Clin Immunol 2021;147:1234–1241.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenerowicz D, Silny W, Dańczak-Pazdrowska A, Polańska A, Osmola-Mańkowska A, Olek-Hrab K. Environmental factors and allergic diseases. Ann Agric Environ Med 2012;19:475–81. [PubMed] [Google Scholar]
- 10.Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines—2016 revision. J Allergy Clin Immunol 2017;140:950–8. [DOI] [PubMed] [Google Scholar]
- 11.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of prenatal supplementation with Vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA 2016;315:362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials 2014;38:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ersfeld DL, Rao DS, Body J-J, Sackrison JL, Miller AB, Parikh N, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON® automated analyzer. Clin Biochem 2004;37:867–74. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 15.Kohler U, Karlson KB, Holm A. Comparing coefficients of nested nonlinear probability models. Stata J Promot Commun Stat Stata 2011;11:420–38. [Google Scholar]
- 16.Wahn U, von Mutius E. Childhood risk factors for atopy and the importance of early intervention. J Allergy Clin Immunol 2001;107:567–74. [DOI] [PubMed] [Google Scholar]
- 17.von Mutius E, Schmid S, PASTURE Study Group. The PASTURE project: EU support for the improvement of knowledge about risk factors and preventive factors for atopy in Europe. Allergy 2006;61:407–13. [DOI] [PubMed] [Google Scholar]
- 18.Bacharier LB. Vitamin D status at birth: an important and potentially modifiable determinant of atopic disease in childhood? J Allergy Clin Immunol 2014;133:154–5. [DOI] [PubMed] [Google Scholar]
- 19.Suh MJ, Park JA, Chang SW, Kim JH, Lee K-H, Hong S-C, et al. Chronological changes in rhinitis symptoms present in school-aged children with allergic sensitization. PLoS One 2019;14:e0210840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Govaere E, Gysel D Van, Massa G, Verhamme KM, Doli E, Baets F De. The influence of age and gender on sensitization to aero-allergens. Pediatr Allergy Immunol 2007;18:671–8. [DOI] [PubMed] [Google Scholar]
- 21.Brustad N, Eliasen AU, Stokholm J, Bønnelykke K, Bisgaard H, Chawes BL. High-dose vitamin D supplementation during pregnancy and asthma in offspring at the age of 6 years. JAMA 2019;321:1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camargo CA, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 2011;127:e180–7. [DOI] [PubMed] [Google Scholar]
- 23.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippilä C, Ahonen S, Nevalainen J, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy 2009;39:875–82. [DOI] [PubMed] [Google Scholar]
- 24.Maslova E, Hansen S, Jensen CB, Thorne-Lyman AL, Strøm M, Olsen SF. Vitamin D intake in mid-pregnancy and child allergic disease - a prospective study in 44,825 Danish mother-child pairs. BMC Pregnancy Childbirth 2013;13:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothers J, Wright A, Stern D, Halonen M, Camargo C. Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization from Tucson, Arizona. J Allergy Clin Immunol 2011;128:1093–1099.e1–1099.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Parental history and the risk for childhood asthma: does mother confer more risk than father? Am J Respir Crit Care Med 1998;158:176–81. [DOI] [PubMed] [Google Scholar]
- 27.Alford SH, Zoratti E, Peterson EL, Maliarik M, Ownby DR, Johnson CC. Parental history of atopic disease: disease pattern and risk of pediatric atopy in offspring. J Allergy Clin Immunol 2004;114:1046–50. [DOI] [PubMed] [Google Scholar]
- 28.Smith M, O’Brien EC, Alberdi G, Geraghty AA, Kilbane M, McKenna MJ, et al. Association between vitamin D status in early pregnancy and atopy in offspring in a vitamin D deplete cohort. Ir J Med Sci 2020;189:563–70. [DOI] [PubMed] [Google Scholar]
- 29.Tian H-Q, Cheng L. The role of vitamin D in allergic rhinitis. Asia Pac Allergy 2017;7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng HM, Kim S, Park GH, Chang SE, Bang S, Won CH, et al. Low vitamin D levels are associated with atopic dermatitis, but not allergic rhinitis, asthma, or IgE sensitization, in the adult Korean population. J Allergy Clin Immunol 2014;133:1048–55. [DOI] [PubMed] [Google Scholar]
- 31.Woon FC, Chin YS, Ismail IH, Abdul Latiff AH, Batterham M, Chan YM, et al. Maternal vitamin D levels during late pregnancy and risk of allergic diseases and sensitization during the first year of life-a birth cohort study. Nutrients 2020;12:2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bener A, Ehlayel MS, Bener HZ, Hamid Q. The impact of Vitamin D deficiency on asthma, allergic rhinitis and wheezing in children: an emerging public health problem. J Family Community Med 2014;21:154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


