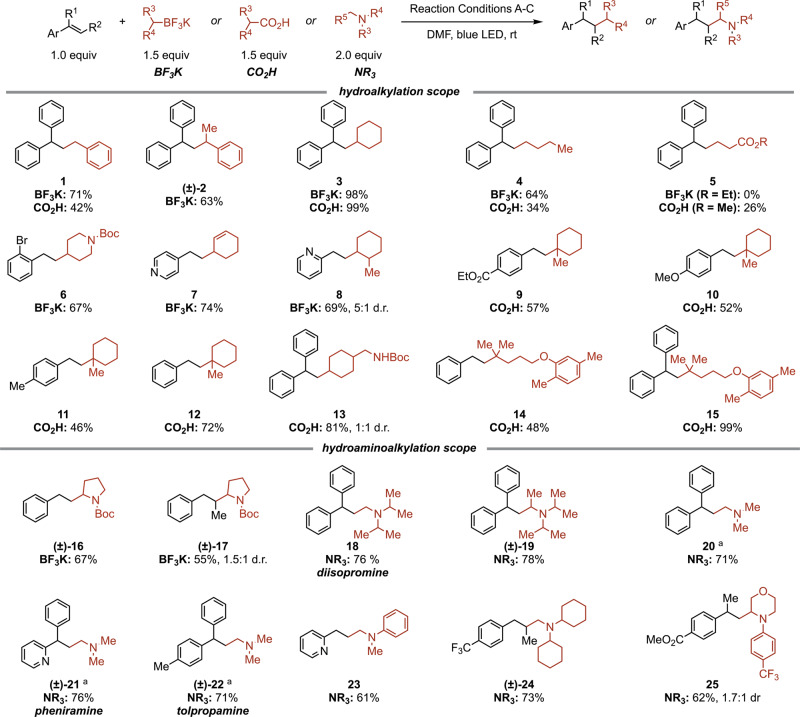
Fig. 3. Scope of hydro(amino)alkylation.
Conditions A: trifluoroborate salt (1.5 equiv), PC (1.0 mol%), 2-methoxylphenol (3.0 equiv), DMF (0.1 M), r.t., 48 h; Conditions B: carboxylic acid (1.5 equiv), PC (1.0 mol%), K3PO4 (1.5 equiv), DMF (0.2 M), r.t., 24 h; Conditions C: tertiary amine (2.0 equiv), PC (0.5 mol%), DMF (0.2 M), r.t., 24 h. a Me3N⋅HCl (3.0 equiv) and DBU (3.0 equiv) were used. See Supplementary Methods for experimental details. d.r. diastereomeric ratio, PC [Ir(dF(CF3)ppy)2(dtbbpy)][PF6].