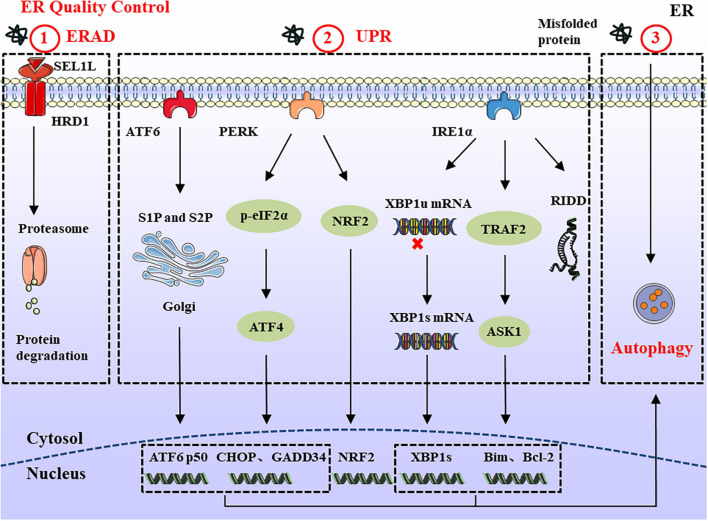
FIGURE 1.
ERQC in Endoplasmic reticulum. ERQC monitors the correct folding of proteins via ERAD, UPR, and autophagy. (1) The soluble protein aggregates are retrotranslocated into cytosol through SEL1L-HRD1, where the ubiquitinated substrates are degraded by the proteasome. (2) When the misfolded proteins exceed ER degradation capacity, it leads to the ER stress response. In order to maintain ER homeostasis, the UPR response is activated which is composed of IRE1α, PERK, and ATF6 branches. The activated IRE1α catalyzes the splicing of XBP1 to generate XBP1s. In addition, IRE1α can also reduce protein synthesis through RIDD, and interacts with TRAF2 to regulate the expression of pro-/anti-apoptotic proteins. PERK reduces protein load by phosphorylating eIF2α and NRF2. Meanwhile, the upregulated ATF4 participates in cell apoptosis and protein synthesis via CHOP and GADD34. ER stress also causes the translocation of ATF6 to the Golgi apparatus, where it is spliced by S1P and S2P proteases, thereby mediating the expression of ERAD and ER chaperones. (3) In the ER stress response, large protein aggregates are degraded through the autophagy-lysosomal pathway.