Abstract
A subset of COVID-19 patients is experiencing secondary immune thrombocytopenia, also called immune thrombocytopenic purpura (ITP) or secondary hemophagocytic lymphohistiocytosis (HLH). The pathogenesis of SARS-CoV-2 associated thrombocytopenia is unknown. Very rare cases of vaccine induced prothrombotic immune thrombocytopenia (VIPIT) are occurring associated with COVID-19 vaccines. COVID-19 VIPIT is associated with autoantibodies targeting platelet factor 4 (PF4) for COVID-19 adenovirus vaccines. Herein, four models for hemophagocytic histocytes contributions to the etiology of thrombocytopenia associated with SARS-CoV-2 are proposed. One of the models proposes potential involvement of hemophagocytic histocytes targeting platelets bound by autoantibodies consistent with observed PF4 autoantibodies in COVID-19 VIPIT.
Keywords: Thrombocytopenia, SARS-CoV-2, COVID-19, Hemophagocytic histiocytes, VIPIT
Introduction
A study of 1,099 COVID-19 patients detected 36.2% with thrombocytopenia (abnormally low levels of platelets) [1]. Platelets, also called thrombocytes, are blood cells that aggregate in response to bleeding injuries to form blood clots together with coagulation factors. COVID-19 causes a spectrum of disease; some patients develop a unique coagulopathy associated with microthrombolic events, elevation of D-dimer levels, and disseminated intravascular coagulopathy (DIC). Platelets can also act like immune cells by binding and internalizing viruses [2], [3]. A hemophagocytic histiocyte is a bloodstream phagocyte (e.g., macrophage). A subset of COVID-19 patients experiencing secondary immune thrombocytopenia (ITP) [4], [5], [6] also called secondary hemophagocytic lymphohistiocytosis (sHLH) [7], [8], reactive HLH, or cerebral venous sinus thrombosis (CVST). ITP overlaps macrophage activation syndrome (MAS). Very rare cases of thrombocytopenia are also associated with two COVID-19 adenoviral vaccines AZD1222 ChAD0x1 (Oxford-AstraZeneca) [9] and Ad26.CoV2.S (Janssen/Johnson & Johnson) [10], [11]. Herein, etiology models for possible hemophagocytic histiocyte contributions to both COVID-19 associated thrombocytopenia and COVID-19 vaccine associated thrombocytopenia are proposed.
The etiology of SARS-CoV-2 associated thrombocytopenia is unknown. Multiple mechanisms are possible including widespread microthrombi formation, immune dysregulation, and autoantibodies binding to platelets. At sites of disrupted endothelium, platelets bind, activate (change shape, secrete chemical messengers, and turn on receptors), followed by aggregation associated with activation of the coagulation cascade associated with fibrin deposition and linking to form microthrombi. Platelets levels can be reduced by formation of multiple microthrombi and also phagocytosis of platelets by macrophages named hemophagocytic histiocytes. Hemophagocytic histiocytes can be visualized with immunohistochemistry combined with in situ hybridization on paraffin-embedded tissue sections from lymph nodes, spleen, liver, and bone marrow [12]. Enrichment of hemophagocytic histocytes is observed in COVID-19 patients and only rarely observed in non-COVID-19-related acute respiratory distress syndrome (ARDS) patients [12]. Observations of scattered hemophagocytic lymphohistiocytes vary between studies with 4 cases associated with spleen but not liver of bone marrow [12], 16 of 17 cases associated with bone marrows [13], and 19 of 19 bone marrows [14]. Scattered macrophages with engulfed erythrocytes, erythroblasts, or lymphocytes are observed [12], [13]. Macrophages containing hemosiderin (suggestive of red blood cell phagocytosis) were also frequently found [12], [13]. Occasionally, multinucleate histiocytes are observed [13]; SARS-CoV-2 infected cells expressing the Spike protein can form multinucleated cells (syncytia) facilitated by cell surface Spike proteins [15]. Platelets can be hyperactivated in COVID-19 [16]. Severe COVID-19 infection is associated with platelet apoptosis induced by antibodies cross-linking Fcγ receptor IIA [17]. Platelet-monocyte aggregate formation is observed in severe COVID-19 patients [18]. Some studies, but not all [19], detect angiotensin-converting enzyme 2 (ACE2) associated with platelets [16], [20]. The SARS-CoV-2 Spike protein has been shown to bind to ACE2 receptors, including platelet ACE2 receptors [20]. In addition, SARS-CoV-2 RNA has been detected within platelets [16]. RNA-seq was performed on RNA from highly purified platelets from 6 non-ICU and 4 ICU SARS-CoV-2 patients and 5 matched healthy donors to find 3,090 differentially expressed genes between non-ICU patients compared to healthy donors and 2,256 differentially expressed genes between ICU patients and health donors [19]. Platelet aggregation in response to low-dose agonists was significantly increased in COVID-19 patients compared to healthy donors [19]. SARS-CoV-2 induces altered platelet gene expression, platelet-leukocyte interactions, platelet-platelet interactions, and increased platelet reactivity [19]. Hemophagocytic histiocytes have been observed in COVID-19 patients [13] and autopsies [12], [13], [14], [21]. Hemophagocytic histiocytes are associated with rare, and often fatal, hemophagocytic syndrome (hemophagocytic lymphohistiocytosis), secondary to other infections (e.g., Epstein-Barr [22] or Dengue Fever [23]), immunodeficiencies [24], cancer [25] or other major events.
Recently, rare cases of thrombocytopenia have been identified following vaccination with encoded SARS-CoV-2 Spike protein. Immune thrombocytopenia has been reported in a case report following COVID-19 vaccination [26]. A man with preexisting thrombocytopenia flared two days after COVID-19 vaccination [27]. Thrombocytopenia is very rare following Pfizer and Moderna SARS-CoV-2 vaccination [28]. In at least 5 countries, at least 13 patients (ages 20 to 50) have symptoms related to widespread blood clots, low platelet counts, and internal bleeding; seven of these patients have died [29], [30]. An association of unusual thrombotic events and thrombocytopenia with autoantibodies targeting platelet factor 4 (PF4) has been advanced in association with the AstraZeneca (AZD1222) ChAdOx1 SARS-CoV-2 vaccine encoding the Spike protein with the suggested name of vaccine induced prothrombotic immune thrombocytopenia (VIPIT) [9], [31], [32]. A 73% morality rate was observed for COVID-19 VIPIT patients with intracranial hemorrhage and platelet counts below 30,000 per cubic millimeter [33]. VIPIT is also been reported in association with both mRNA COVID-19 Spike vaccines [34]. PF4, also called CXCL4, is a tetrameric chemokine stored in platelet alpha-granules. Activated platelets release PF4 which binds polyanions with high affinity [35] and surface proteoglycans. PF4 plays an important role in heparin-induced thrombocytopenia (HIT) [36], [37]. For COVID-19 adenovirus vaccines, activation of platelets is possible by adenovirus [38]. Greinacher et al. recommend non-heparin anticoagulants and proposed evaluation of high-dose immunoglobulin as possible treatments [9].
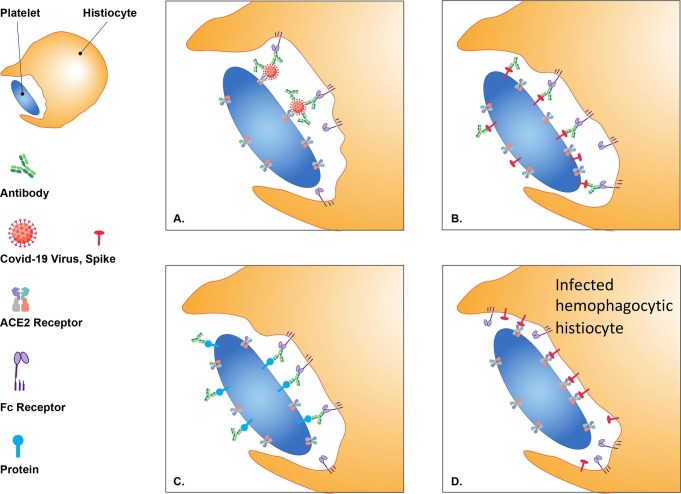
Herein, I propose four models for hemophagocytic histiocytes contributions to the etiology of thrombocytopenia associated with SARS-CoV-2.
-
1.
The first model proposes that histiocytes are targeting platelets with ACE2 externally bound virions (Fig. 1 -A).
-
2.
The second model proposes that histiocytes are targeting platelets expressing viral proteins (Fig. 1-B).
-
3.
A striking loss of germinal centers in lymph nodes and spleen was observed in COVID-19 patients [39]; this may contribute to expansion of possible autoantibodies that bind platelets. The third model proposes that hemophagocytic histiocytes target platelets bound by autoantibodies (Fig. 1-C). Thrombocytopenia associated with PF4-autoantibodies is consistent with this model (Fig. 1-C).
-
4.
Most monocytes and macrophages do not express the ACE2 protein. I previously proposed expanded cellular tropism to phagocytic cells binding SARS-CoV-2 with Fc receptors [40]. This has been observed in COVID-19 patients with infected macrophages [41], [42] and monocytes [43]. SARS-CoV-2-infected cells express the Spike protein on their surface [15]. The fourth model proposes that infected monocytes and macrophages express Spike proteins on their surface and target platelets by binding ACE2 to become hemophagocytic histiocytes (Fig. 1-D).
Fig. 1.
Thrombocytopenia Models (A) Hemophagocytic histocytes engulfing platelets with SARS-CoV-2 viruses bound to ACE2 receptors by Fc receptor binding to SARS-CoV-2 antibodies; (B) Hemophagocytic histocytes engulfing platelets with surface expressed SARS-CoV-2 proteins by Fc receptor binding to SARS-CoV-2 antibodies; (C) Hemophagocytic histocytes engulfing platelets by Fc receptor binding to platelet bound autoantibodies (amplified in COVID-19); and (D) Hemophagocytic histocytes engulfing platelets by surface expressed Spike proteins binding to platelet ACE2 receptors with either SARS-CoV-2 infection of hemophagocytic histocytes or vaccine encoded Spike proteins.
These proposed models can be evaluated to determine thrombocytopenia pathogenesis in COVID-19 patients. To test the first model, isolated hemophagocytic histiocytes can be evaluated for histiocyte phagocytosis of platelets alone (little or no phagocytosis of platelets) compared to platelets mixed with SARS-CoV-2 virions (phagocytosis of platelets with SARS-CoV-2 virions binding to platelet ACE2 receptors); essentially, comparing histiocyte phagocytosis of COVID-19 patient platelets compared to platelets from non-COVID-19 controls. An antibody to the Spike protein may be sufficient to evaluate the second model for platelets expressing viral proteins. Enzyme-linked immunosorbent assays (ELISA) [9], [31] or an antibody to the Fc or other constant region of antibodies could detect autoantibodies binding platelets to evaluate the third model. The fourth model can be evaluated with electron microscope image analysis of hemophagocytic histiocytes for coronavirus-like particles [13], [44]. Blocking the binding of Spike with ACE2 may provide therapeutic benefits against model 1 (blocking platelet ACE2 binding virion) and model 4 (blocking platelet ACE2 binding histiocyte expressed Spike); the ability to block at therapeutic dosages is unknown. Intravenous gamma globulin (IVIG) may provide therapeutic benefits against models 1, 2, and 3 [9]; essentially diluting Fcγ receptor mediated binding of antibodies and Fcγ receptor mediated activation of platelets observed in VIPIT [9].
Summary
Four etiology models for hemophagocytic histiocytes contributions to thrombocytopenia associated with SARS-CoV-2 are proposed. In addition, one of these models is consistent with hemophagocytic histocytes contributing to VIPIT associated with SARS-CoV-2 adenoviral vaccines AZD1222 ChAD0x1 and Ad26.CoV2.S (Janssen/Johnson & Johnson).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The author acknowledges Irene Stapleford for graphic art assistance.
DISTRIBUTION STATEMENT A. Approved for public release. Distribution is unlimited.
This material is based upon work supported by the Under Secretary of Defense for Research and Engineering under Air Force Contract No. FA8702-15-D-0001. Any opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the United States Air Force
References
- 1.Guan W.-j., Ni Z.-y., Hu Y., Liang W.-h., Ou C.-q., He J.-x., et al. novel coronavirus infection in China. N Engl J Med. 2019;2020(382):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koupenova M., Corkrey H.A., Vitseva O., Manni G., Pang C.J., Clancy L., et al. The role of platelets in mediating a response to human influenza infection. Nat Commun. 2019;10(1) doi: 10.1038/s41467-019-09607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee M., Huang Y., Joshi S., Popa G.J., Mendenhall M.D., Wang Q.J., et al. Platelets Endocytose Viral Particles and Are Activated via TLR (Toll-Like Receptor) Signaling. Arterioscler Thromb Vasc Biol. 2020;40(7):1635–1650. doi: 10.1161/ATVBAHA.120.314180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsao H.S., Chason H.M., Fearon D.M. Immune Thrombocytopenia (ITP) in a Pediatric Patient Positive for SARS-CoV-2. Pediatrics. 2020;146(2):e20201419. doi: 10.1542/peds.2020-1419. [DOI] [PubMed] [Google Scholar]
- 5.Merli M., Ageno W., Sessa F., Salvini M., Caramazza D., Mora B., et al. Recurrence of immune thrombocytopenia at the time of SARS-CoV-2 infection. Ann Hematol. 2020;99(8):1951–1952. doi: 10.1007/s00277-020-04130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mei H., Luo L., Hu Y. Thrombocytopenia and thrombosis in hospitalized patients with COVID-19. J Hematol Oncol. 2020;13(1):161. doi: 10.1186/s13045-020-01003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Núñez-Torrón C, Ferrer-Gómez A, Moreno Moreno E, Pérez-Mies B, Villarrubia J, Chamorro S, et al. Secondary haemophagocytic lymphohistiocytosis in COVID-19: correlation of the autopsy findings of bone marrow haemophagocytosis with HScore. J Clin Pathol. 2021. [DOI] [PubMed]
- 8.Lima R., Filho C.C., Ferreira Filho C.M., Vaisman M., Cossenza A., Rebello C.P., et al. Hemophagocytic syndrome and COVID-19. Respir Med Case Rep. 2020;31:101162. doi: 10.1016/j.rmcr.2020.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muir K.-L., Kallam A., Koepsell S.A., Gundabolu K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N Engl J Med. 2021;384(20):1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448-56. [DOI] [PMC free article] [PubMed]
- 12.Prilutskiy A, Kritselis M, Shevtsov A, Yambayev I, Vadlamudi C, Zhao Q, et al. SARS-CoV-2 Infection-Associated Hemophagocytic Lymphohistiocytosis. Am J Clin Pathol. 2020;154(4):466-74. [DOI] [PMC free article] [PubMed]
- 13.Prieto-Pérez L., Fortes J., Soto C., Vidal-González Á., Alonso-Riaño M., Lafarga M., et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod Pathol. 2020;33(11):2139–2146. doi: 10.1038/s41379-020-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris CK, Hung YP, Nielsen GP, Stone JR, Ferry JA. Bone Marrow and Peripheral Blood Findings in Patients Infected by SARS-CoV-2. Am J Clin Pathol. 2021:aqaa274. [DOI] [PMC free article] [PubMed]
- 15.Buchrieser J., Dufloo J., Hubert M., Monel B., Planas D., Rajah M.M., et al. Syncytia formation by SARS-CoV-2-infected cells. EMBO J. 2020;39(23) doi: 10.15252/embj.2020106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaid Y., Puhm F., Allaeys I., Naya A., Oudghiri M., Khalki L., et al. Platelets can contain SARS-CoV-2 RNA and are hyperactivated in COVID-19. Circ Res. 2020;127(11):1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Althaus K, Marini I, Zlamal J, Pelzl L, Singh A, Häberle H, et al. Antibody-induced procoagulant platelets in severe COVID-19 infection. Blood. 2021;137(8):1061-71. [DOI] [PMC free article] [PubMed]
- 18.Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330-41. [DOI] [PMC free article] [PubMed]
- 19.Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317-29. [DOI] [PMC free article] [PubMed]
- 20.Zhang S.i., Liu Y., Wang X., Yang L.i., Li H., Wang Y., et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13(1) doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, et al. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv (Preprint). 2020.
- 22.Marsh R.A. Epstein-Barr Virus and Hemophagocytic Lymphohistiocytosis. Front Immunol. 2018;8:1902. doi: 10.3389/fimmu.2017.01902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray S., Kundu S., Saha M., Chakrabarti P. Hemophagocytic syndrome in classic dengue Fever. J Glob Infect Dis. 2011;3(4):399–401. doi: 10.4103/0974-777X.91068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bode S.F., Ammann S., Al-Herz W., Bataneant M., Dvorak C.C., Gehring S., et al. The syndrome of hemophagocytic lymphohistiocytosis in primary immunodeficiencies: implications for differential diagnosis and pathogenesis. Haematologica. 2015;100(7):978–988. doi: 10.3324/haematol.2014.121608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H., Xiong L., Tang W., Zhou Y., Li F. A systematic review of malignancy-associated hemophagocytic lymphohistiocytosis that needs more attentions. Oncotarget. 2017;8(35):59977–59985. doi: 10.18632/oncotarget.19230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarawneh O., Tarawneh H. Immune thrombocytopenia in a 22-year-old post Covid-19 vaccine. Am J Hematol. 2021 doi: 10.1002/ajh.26106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leshan B. Man says his rare blood disorder flared after COVID-19 vaccination 2021 Available from: https://www.khou.com/article/news/health/coronavirus/vaccine/rockville-covid-vaccine-serious-side-effects-rare-blood-disorder-itp-thrombocytopenia/65-37336643-c641-49e0-8f52-1808513f42d9.
- 28.Lee E.-J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D., et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021 doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel G., Kupferschmidt K. ‘It’s a very special picture’. Why vaccine safety experts put the brakes on AstraZeneca’s COVID-19 vaccine. Science. 2021 [Google Scholar]
- 30.Bjørnstad-Tuveng T.H., Rudjord A., Anker P. Fatal cerebral haemorrhage after COVID-19 vaccine. Tidsskr Nor Legeforen. 2021 doi: 10.4045/tidsskr.21.0312. [DOI] [PubMed] [Google Scholar]
- 31.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scully M., Singh D., Lown R., Poles A., Solomon T., Levi M., et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavord S, Scully M, Hunt BJ, Lester W, Bagot C, Craven B, et al. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed]
- 34.Kuter D.J. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br J Haematol. 2021 doi: 10.1111/bjh.17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rauova L, Poncz M, McKenzie SE, Reilly MP, Arepally G, Weisel JW, et al. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood. 2005;105(1):131-8. [DOI] [PubMed]
- 36.Ziporen L, Li ZQ, Park KS, Sabnekar P, Liu WY, Arepally G, et al. Defining an Antigenic Epitope on Platelet Factor 4 Associated With Heparin-Induced Thrombocytopenia. Blood. 1998;92(9):3250-9. [PubMed]
- 37.Visentin G.P., Ford S.E., Scott J.P., Aster R.H. Antibodies from patients with heparin-induced thrombocytopenia/thrombosis are specific for platelet factor 4 complexed with heparin or bound to endothelial cells. J Clin Invest. 1994;93(1):81–88. doi: 10.1172/JCI116987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Othman M., Labelle A., Mazzetti I., Elbatarny H.S., Lillicrap D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109(7):2832–2839. doi: 10.1182/blood-2006-06-032524. [DOI] [PubMed] [Google Scholar]
- 39.Kaneko N., Kuo H.-H., Boucau J., Farmer J.R., Allard-Chamard H., Mahajan V.S., et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell. 2020;183(1):143–157.e13. doi: 10.1016/j.cell.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricke D.O. Two Different Antibody-Dependent Enhancement (ADE) Risks for SARS-CoV-2 Antibodies. Front Immunol. 2021;12:443. doi: 10.3389/fimmu.2021.640093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martines R.B., Ritter J.M., Matkovic E., Gary J., Bollweg B.C., Bullock H., et al. Pathology and Pathogenesis of SARS-CoV-2 Associated with Fatal Coronavirus Disease. United States Emerg Infect Dis. 2020;26(9):2005–2015. doi: 10.3201/eid2609.202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Junqueira C, Crespo Â, Ranjbar S, Ingber J, Parry B, Ravid S, et al. SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release. medRxiv (Preprint). 2021:2021.03.06.21252796.
- 44.Colmenero I., Santonja C., Alonso‐Riaño M., Noguera‐Morel L., Hernández‐Martín A., Andina D., et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183(4):729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]