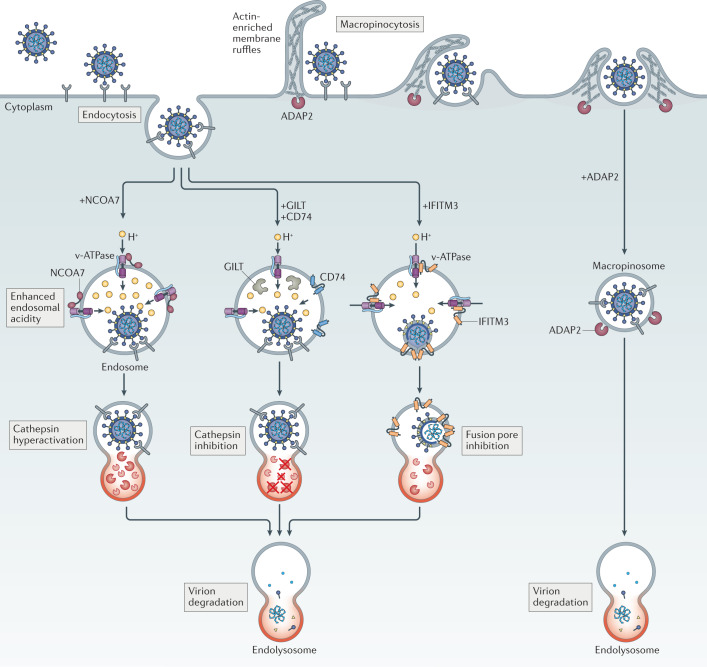
Fig. 4. Intrinsic inhibitors of virus entry promote virion degradation in endolysosomes via distinct mechanisms.
The antiviral activities exhibited by nuclear receptor co-activator protein 7 (NCOA7), interferon-induced transmembrane (IFITM) proteins, interferon-γ-inducible lysosomal thiol reductase (GILT), CD74 and ARFGAP with dual pleckstrin homology domain-containing protein 2 (ADAP2) act against viruses entering cells through pH-dependent fusion in endosomes. Whereas NCOA7 and IFITM3 have been reported to interact with vacuolar ATPase (v-ATPase), only NCOA7 may increase the acidity (lower the pH) of the endosomal lumen. NCOA7-mediated acidification of endosomes is associated with enhanced cathepsin activity in endolysosomes, and this elevated level of proteolytic activity may promote virion degradation before virus–cell fusion occurs. IFITM3, on the other hand, inhibits membrane fusion itself, resulting in endosomal sequestration of virions that are eventually degraded in endolysosomes. This latter effect results from the ability of IFITM3 to promote endolysosomal delivery of viral and cellular cargo, a function that is not yet mechanistically understood. GILT and CD74 are believed to enforce endosomal sequestration of viruses as well, but not by inhibiting membrane fusion — they inhibit the activity of endolysosomal cathepsins, proteases that cleave some viral glycoproteins and render them competent for fusion. ADAP2 promotes internalization of virions by macropinocytosis, bypassing their preferred sites for pH-dependent fusion and resulting in their accelerated disposal in endolysosomes.