Abstract
Objective
The goal of this randomized, double-blinded, placebo-controlled clinical trial was to investigate the therapeutic efficacy of oral 25-hydroxyvitamin D3 (25(OH)D3) in improving vitamin D status in vitamin D–deficient/vitamin D–insufficient patients infected with the SARS-CoV-2 (COVID-19) virus.
Methods
This is a multicenter, randomized, double-blinded, placebo-controlled clinical trial. Participants were recruited from 3 hospitals that are affiliated to [Institution Blinded for Review] and [Institution Blinded for Review].
Results
A total 106 hospitalized patients who had a circulating 25(OH)D3 concentration of <30 ng/mL were enrolled in this study. Within 30 and 60 days, 76.4% (26 of 34) and 100% (24 of 24) of the patients who received 25(OH)D3 had a sufficient circulating 25(OH)D3 concentration, whereas ≤12.5% of the patients in the placebo group had a sufficient circulating 25(OH)D3 concentration during the 2-month follow-up.
We observed an overall lower trend for hospitalization, intensive care unit duration, need for ventilator assistance, and mortality in the 25(OH)D3 group compared with that in the placebo group, but differences were not statistically significant. Treatment with oral 25(OH)D3 was associated with a significant increase in the lymphocyte percentage and decrease in the neutrophil-to-lymphocyte ratio in the patients. The lower neutrophil-to-lymphocyte ratio was significantly associated with reduced intensive care unit admission days and mortality.
Conclusion
Our analysis indicated that oral 25(OH)D3 was able to correct vitamin D deficiency/insufficiency in patients with COVID-19 that resulted in improved immune function by increasing blood lymphocyte percentage. Randomized controlled trials with a larger sample size and higher dose of 25(OH)D3 may be needed to confirm the potential effect of 25(OH)D3 on reducing clinical outcomes in patients with COVID-19.
Key words: COVID-19, 25-hydroxyvitamin D3, viral infection, supplementation, lymphocyte, vitamin D deficiency
Abbreviations: CI, confidence interval; ICU, intensive care unit; IQR, interquartile range; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; 1,25(OH)2D, 1,25-dihydroxyvitamin D; RCT, randomized controlled trials; SMD, standardized mean difference; 25(OH)D3, 25-hydroxyvitamin D3
Introduction
COVID-19 is a respiratory and systemic disorder caused by SARS-CoV-2. As of May 13, 2021, this pandemic has caused a total of 139 933 765 confirmed cases and 3 346 652 deaths worldwide.1 Higher mortality and morbidity rates have been observed in patients with severe pneumonia and in some case associated with multiorgan failure and occur in approximately half of hospitalized patients.2 , 3 A recent large meta-analysis on more than 10 000 subjects demonstrated that vitamin D supplementation had a protective role in acute respiratory infections in adults.4 Due to these findings, an important role for vitamin D has been suggested in the treatment or prevention of COVID-19.
Recently, observational studies have reported a link between vitamin D deficiency and morbidity and mortality associated with COVID-19.5, 6, 7 However, few trials have been conducted to determine if the improvement in vitamin D status during hospitalization provided any benefit.8 , 9 The most common form of dietary vitamin D supplementation used today is cholecalciferol or vitamin D3. It is hypothesized that increasing the serum 25-hydroxyvitamin D3 (25[OH]D3) concentrations above 30 ng/mL and in the range of 40 to 60 ng/mL may noticeably reduce the severity and mortality of various viral diseases, including COVID-19.10, 11, 12 The Endocrine Society guidelines recommend for adults that 1500 to 2000 IU/day of vitamin D may be required to increase the blood concentration of 25(OH)D3 consistently above 30 ng/mL (75 nmol/L).13 It takes at least 3 to 4 weeks of 1000 IU/day of vitamin D3 to reach a plateau in the range of 30 ng/mL in the circulating serum 25(OH)D3 concentrations.14 , 15
As an alternative strategy to increase the serum 25(OH)D3 concentrations in vitamin D–deficient adults, oral supplementation of 25(OH)D3 (calcifediol) has been suggested.15 When vitamin D3 is ingested, it is incorporated into chylomicrons and enters the lymphatic system. The chylomicrons then enter the bloodstream via the superior cava. Most of the vitamin D is incorporated into the body fat. Vitamin D3 in the circulation and the vitamin D3 that is slowly released from the body fat into the circulation are converted in the liver to 25(OH)D3. This is the likely explanation for why it takes approximately 3 to 4 weeks to achieve a steady state concentration of 25(OH)D3.14 , 16 , 17 25(OH)D3 is more hydrophilic, and therefore, after ingestion, it is absorbed into the venous portal system, thereby rapidly increasing the circulating 25(OH)D3 concentrations. It was reported that orally administered 20 μg of 25(OH)D3 compared with 800 IU (20 μg) of vitamin D3 was significantly more efficient and rapid in increasing the serum 25(OH)D3 concentrations in healthy postmenopausal women into a desirable range of at least 30 ng/mL. The rapid increase in the serum 25(OH)D3 concentrations was related to a decrease in innate immunity markers including eotaxin, interleukin 12, monocyte chemoattractant protein-1, and macrophage inflammatory protein-1 beta.16 After oral consumption of 25(OH)D3, the major circulating form of vitamin D, it is converted in the kidneys to 1,25-dihydroxyvitamin D (1,25[OH]2D) through CYP27B1 (1-α-hydroxylase) and enters circulation and interacts with the vitamin D receptor for the purpose of regulating calcium and bone metabolism.18 , 19 The activated monocytes and macrophages express CYP27B1, producing 1,25(OH)2D from circulating 25(OH)D3, inducing antibacterial agents.18 , 19
Thus, 25(OH)D3 consumption would improve vitamin D status more rapidly and be more available for target immune cells for fighting with coronavirus. We aimed to investigate the potential therapeutic benefit of rapidly increasing the circulating serum 25(OH)D3 concentrations with orally administered 25(OH)D3 in patients with COVID-19.
Materials and Methods
Study Design and Participants
This multicenter clinical trial was designed as a randomized, double-blinded, placebo-controlled trial. Participants were recruited from hospitals that are affiliated to Tehran University of Medical Sciences (TUMS) (Ziaeian, and Dr Shariati hospitals), and Shahid Beheshti University of Medical Sciences (Shohadaye Pakdasht Hospital).
The recruitment was started on May 2020, and the study ran until October 2020. All measurements were analyzed at the admission date, at the release date, and after the first and second months of follow-up.
COVID-19 (SARS-CoV-2) was diagnosed by acute respiratory tract infection symptoms (eg, fever, cough, and dyspnea) with no other etiology that fully explained the clinical presentation. The diagnosis was supported by chest computed tomography scan findings compatible with COVID-19 and/or a definitive diagnosis of COVID-19 with real-time polymerase chain reaction.
Inclusion and Exclusion Criteria
The inclusion criteria of study subjects were as follows:
-
1.
Older than 18 years old
-
2.
No medications or disorders that would affect vitamin D metabolism
-
3.
Vitamin D deficiency/insufficiency (25[OH]D3 concentration of <30 ng/mL)
-
4.
Ability and willingness to give informed consent and comply with protocol requirements
The exclusion criteria were as follows:
-
1.
Pregnant or lactating women
-
2.
Severe underlying diseases, such as advanced malignant tumor and end-stage lung disease
-
3.
Chronic hepatic dysfunction and intestinal malabsorption syndromes including inflammatory bowel disease
-
4.
Ongoing treatment with pharmacologic doses of vitamin D, vitamin D metabolites, or analogs
-
5.
Supplementation with over-the-counter formulations of vitamin D2 or vitamin D3
-
6.
Use of tanning bed or artificial ultraviolet exposure within the last 2 weeks
-
7.
Consuming medication affecting vitamin D metabolism or absorption (anticonvulsants, antituberculosis medication glucocorticoids, HIV medications and cholestyramine)
-
8.
History of an adverse reaction to orally administered vitamin D, vitamin D metabolites, or analogs
-
9.
History of an elevated serum calcium concentration of >10.6 mg/dL that was corrected for albumin concentration or subjects with a history of hypercalciuria and kidney stones
-
10.
History of conditions that could lead to high serum calcium concentrations, such as sarcoidosis, tuberculosis, and some lymphomas associated with activated macrophages, which increase the production of 1,25(OH)2D
-
11.
Inability to give informed consent
Recruitments and Inform Consent Process
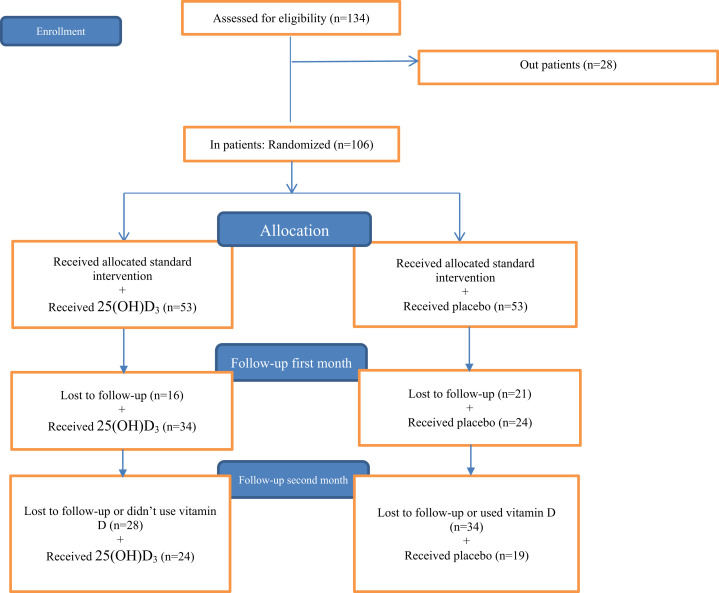
Eligible subjects were enrolled in the study after consenting process to provide a blood sample to evaluate the serum 25(OH)D3 concentration. All participants with vitamin D deficiency/insufficiency (25[OH)D3 concentration of <30 ng/mL) were randomized.13 The study flow diagram is shown in Figure 1 .
Fig. 1.
Flowchart of the study participants.
Once the subjects were determined to be eligible, they were presented with the consent form by trained research nurses. The participants also received information sheets. Research nurses were discussing the trial with subjects in light of the information sheets. In addition, there was a plan to provide medical advice or counseling to subjects who were screened and met the 25(OH)D3 concentration of <30 ng/mL criteria who decided not to participate in the study.
Randomization
All participants in a stratified random sampling method were recruited in the 25(OH)D3 or placebo group with a ratio of 1:1. The clinical coordinator determined this using a computer-generated randomization program. Subjects in the treatment group (n = 53) received 25(OH)D3, and the nontreatment group (n = 53) received placebo. The randomization time was on the day of admission to take oral 25(OH)D3 or placebo.
Intervention
The 25(OH)D3 (Calcifediol) and placebo capsules were generously provided by Carbogen Amcis BV, a company belonging to the Dishman Group. 25(OH)D3 was formulated in medium-chain fatty acids and then encapsulated. The placebo contained the same amount of medium-chain fatty acids and was also encapsulated. The participants received randomly either a bottle containing 30 capsules of 25(OH)D3 or placebo in their first visit and then again 30 days later. The bottles were returned to be counted at each visit. The dose of 25(OH)D3 was 25 μg administered orally once daily. At the time that we initiated our clinical research trial, there was no evidence to suggest that a higher dose of vitamin D3 or 25(OH)D3 would be more effective in reducing the risk of morbidity and mortality in COVID-19. Because of safety concerns, we used a dose of 25(OH)D3 that was equivalent to approximately 3000 to 6000 IU per day of vitamin D3. The study was suspended if the serum calcium concentration was consistently above the normal range or the serum 25(OH)D3 concentration was above 100 ng/mL. During the trial, there were not any patients with serum calcium concentrations of >10.6 mg/dL or serum 25(OH)D3 concentrations of >100 ng/mL. There were not any adverse reactions reported by participants during the consumption of oral 25(OH)D3 or the placebo.
Blinding
All subjects at the clinical departments were blinded to the trial intervention allocation. The main outcomes were evaluated by physicians.
Compliance
The subjects were followed up weekly by phone to remind study participants to use their study medication and monitor dosing compliance as well as to ask about their medical symptoms. The subjects were asked to return the first and second bottles of the study medication after the first and second months of hospital admission date, respectively, for recounting to evaluate their compliance and assessing biochemical tests as well as serum 25(OH)D3 concentrations.
Study Outcomes
-
1.
Severity of COVID-19 (SARS-CoV-2) infection: percentage of mild, moderate, and severe forms of COVID-19 based on the World Health Organization criteria
-
2.
Length of stay in the hospital: days from admission to discharge from the hospital
-
3.
Oxygen support: percentage of patients with COVID-19 who need oxygen support
-
4.
Death: rate of death due to COVID-19 during the study
-
5.
Lymphocyte count and percentage
-
6.
Serum 25(OH)D3 concentrations at baseline and after 30 and 60 days of starting oral 25(OH)D3 or placebo (first and second months of follow-up)
Study Measurements
Data included the following information: (1) demographic information (age, sex, and body mass index), (2) smoking habit, (3) medical history, (4) principal clinical symptoms and their onset time, (5) real-time polymerase chain reaction results, (6) radiological findings, (7) laboratory findings, (8) comorbidities, and (9) disease progression.
Laboratory examination at the time of admission to the hospital or soon thereafter included a complete blood count, blood biochemistries (total 25[OH]D3, calcium, phosphorus, magnesium, sodium, potassium, alanine aminotransferase, aspartate aminotransferase, creatine kinase, lactate dehydrogenase [LDH], creatine phosphokinase, C-reactive protein, procalcitonin, erythrocyte sedimentation rate, bilirubin), and arterial blood gas (partial pressure of carbon dioxide, partial pressure of oxygen, bicarbonate, and pH). Total serum 25(OH)D3 was measured by high-performance liquid chromatography. The method of high-performance liquid chromatography was described in our recent study.20
Statistical Analysis
Data were analyzed using SPSS statistical software (version 20). Continue variables are presented as mean (standard deviation) for normally distributed data or median (interquartile range [IQR]) for nonnormally distributed data. The parametric and nonparametric tests, including the independent t test and Mann-Whitney U test, were used to compare differences between continuous variables where appropriate. Categorical variables are presented as percentage. The chi-square or Fisher exact test was used to examine the percentage differences in the sign and symptom, need for mechanical ventilation, need for intensive care, and hospital mortality rates in the treated and placebo groups. The standardized mean difference (SMD) was used to express the size of the intervention effect on increasing the circulating vitamin D concentrations in the treatment group compared with the placebo group. The logistic regression model was used to consider an independent association of the neutrophil-to-lymphocyte ratio (NLR) and clinical outcomes.
All tests were 2-sided, and P values of <.05 were considered significant.
Safety
Since the clinical trial was designed as a minimal risk, a formal committee for data monitoring was not required. However, potential toxicity was monitored in 2 steps, the first and second months of follow-up, for serum concentrations of 25(OH)D3, calcium, albumin, and creatinine. We monitored for early signs and symptoms of vitamin D toxicity and hypercalcemia in all participants. The subjects were followed up weekly by phone to ask about their medical symptoms.
Ethics and Dissemination
This clinical trial was conducted according to the Declaration of Helsinki. The Ethics Committee of the [Blinded] University of Medical Sciences approved this clinical trial (approval number: IR.TUMS.VCR.REC.1399.061). A SPIRIT checklist is available for this protocol. This clinical trial has been registered at ClinicalTrials.gov with the identifier [Number Blinded for Review]. Participants signed informed consent.
Results
Based on the inclusion criteria, a total of 106 vitamin D–deficient/vitamin D–insufficient hospitalized patients were enrolled in this study: 53 in the placebo group and 53 in the treatment group with 25(OH)D3 (Fig. 1).
All patients received the same standard care; a combination of hydroxychloroquine, azithromycin, and, for patients with pneumonia, ceftriaxone was used. During hospitalization, all participants received 30 capsules (first box) of 25(OH)D3 or placebo to take in the hospital and continued at home if they were discharged earlier. After 30 days of starting 25(OH)D3 or placebo, all participants who were discharged from the hospital and who visited the outpatient COVID-19 centers were recruited to take the second box of capsules; 38 in the treatment group and 31 in the placebo group. For the second month of follow-up, 24 patients in the treatment group and 19 in the placebo group returned to the outpatient COVID-19 centers. Potential toxicity was monitored at each follow-up visit (after 30 and 60 days of starting 25(OH)D3 or placebo) for serum concentrations of calcium, albumin, creatinine (Supplementary Table 1) and 25(OH)D. Concern about COVID-19 reinfection was the main reason of loss to follow-up.
Baseline and Clinical Characteristics
The baseline and clinical characteristics of the included participants are summarized in Table 1 . The mean age of all participants was 49.1 ± 14.1 years (48.9 ± 13.6 years in men and 49.5 ± 14.9 years in women). There were no significant age and sex differences in each group. There were not significant differences in hematologic and biochemical tests and serologic markers (Table 1).
Table 1.
Demographic Characteristics and Biochemical Tests at Baseline
| Baseline characteristics | N (25[OH]D3/placebo) | 25(OH)D3 | Placebo | P value |
|---|---|---|---|---|
| Age (years) | 53/53 | 50 ± 15 | 49 ± 13 | .6 |
| Sex (female) | 53/53 | 41% (22) | 38% (20) | .7 |
| SpO2 (%) | 52/52 | 90 ± 5 | 89 ± 7 | .6 |
| Heart rate (beats/min) | 50/49 | 89 ± 11 | 88 ± 15 | .8 |
| Respiratory rate (breaths/min) | 51/49 | 19.5 ± 3 | 20 ± 4.5 | .4 |
| Temperature (° C) | 51/48 | 37 ± 0.9 | 37 ± 0.6 | .7 |
| Systolic blood pressure (mm Hg) | 51/49 | 117 ± 16 | 120 ± 16 | .4 |
| Diastolic blood pressure (mm Hg) | 51/49 | 73 ± 11 | 75 ± 10 | .4 |
| Chronic disorder | 53/53 | 55% (29) | 45% (24) | .3 |
| Smoking | 50/49 | 12% (6) | 9% (4) | .6 |
| CT involvement—bilateral | 42/39 | 88% (37) | 89% (35) | .3 |
| CT severity | 37/33 | |||
| Moderate and severe | 65% (24) | 73% (24) | .5 | |
| None and mild | 35% (13) | 27% (9) | ||
| Body mass index (kg/m2) | 53/53 | 29 ± 6 | 29 ± 5.5 | .8 |
| Hematology tests | ||||
| WBC (×1000 /mL ) | 53/52 | 6.9 ± 3.3 | 7.4 ± 3.9 | .6 |
| RBC (million/mL) | 53/53 | 4.6 ± 0.7 | 4.6 ± 0.8 | .5 |
| Hemoglobin (g/dL) | 53/53 | 13 ± 1.8 | 13.4 ± 2.3 | .3 |
| MCV (fL) | 53/53 | 84 ± 7.5 | 85 ± 8 | .6 |
| Platelet (×1000 /mL) | 53/53 | 202 ± 85 | 213 ± 104 | .6 |
| Neutrophils (%) | 52/53 | 71 ± 13 | 73 ± 12 | .6 |
| Lymphocyte (%) | 52/53 | 25 ± 12 | 23 ± 11 | .4 |
| Blood biochemistry | ||||
| 25(OH)D3 (ng/mL) | 53/53 | 19 ± 8 | 18 ± 8 | .7 |
| Ln.ESR.1h (mm/h) | 48/45 | 3.5 ± 1.0 | 3.5 ± 0.6 | .6 |
| Ln. blood urea nitrogen (mg/dL) | 53/51 | 3.3 ± 0.5 | 3.4 ± 0.5 | .6 |
| Creatinine (mg/dL) | 53/51 | 1.1 ± 0.3 | 1.0 ± 0.3 | .4 |
| Ln. AST (U/L) | 35/33 | 3.4 ± 0.5 | 3.63 ± 0.6 | .1 |
| Ln. ALT (U/L) | 35/33 | 3.5 ± 0.5 | 3.64 ± 0.6 | .4 |
| ALP (U/L) | 33/31 | 140 (110) | 133 (146) | .1 |
| Phosphorus (mg/dL) | 30/28 | 3.6 ± 0.7 | 3.8 ± 0.6 | .4 |
| Calcium (mg/dL) | 34/32 | 8.7 ± 0.5 | 8.6 ± 0.6 | .3 |
| Sodium (mEq/L) | 51/51 | 137 ± 4 | 138 ± 4 | .2 |
| Potassium (mEq/L) | 51/52 | 4.2 ± 0.5 | 4.2 ± 0.5 | .9 |
| Magnesium (mg/dL) | 37/30 | 2.0 ± 0.2 | 2.1 ± 0.3 | .6 |
| Albumin (gr/dL) | 31/27 | 4.4 ± 0.4 | 4.0 ± 0.7 | .02 |
| Bilirubin total (mg/dL) | 33/31 | 0.7 (0.6) | 0.9 (0.5) | .7 |
| Bilirubin direct (mg/dL) | 32/31 | 0.2 (0.1) | 0.2 (0.2) | .8 |
| Ln. CPK (U/L) | 37/31 | 4.8 ± 0.8 | 4.8 ± 0.8 | .8 |
| Ln. LDH (U/L) | 44/42 | 6.2 ± 0.5 | 6.3 ± 0.5 | .3 |
| Venous blood gases | ||||
| pH | 32/27 | 7.4 ± 0.1 | 7.4 ± 0.1 | .2 |
| PCO2 (mm Hg) | 32/26 | 40.5 ± 7 | 39 ± 7 | .4 |
| PO2 (mm Hg) | 31/26 | 34 ± 15 | 34 ± 10 | .9 |
| Bicarbonate (mmol/L) | 32/27 | 24 ± 3 | 24 ± 3 | .9 |
| Serology test | ||||
| C-reactive protein (qualitative) | 43/44 | |||
| Negative | 32.6% (14) | 27.3% (12) | .4 | |
| +1 | 27.9% (12) | 31.8% (14) | ||
| +2 | 18.6% (8) | 29.5% (13) | ||
| +3 | 20.9% (9) | 11.4% (5) | ||
Abbreviations: ALT = alanine aminotransferase; ALP = alkaline phosphatase; AST = aspartate aminotransferase; CPK = creatine phosphokinase; CT = computed tomography; ESR = erythrocyte sedimentation rate; h = hour; LDH = lactate dehydrogenase; min = minute; MCV = mean corpuscular volume; PCO2 = partial pressure of carbon dioxide; PO2 = partial pressure of oxygen; RBC = red blood cell; SpO2= oxygen saturation; 25(OH)D3 = 25-hydroxyvitamin D3; WBC = white blood cell.
Numerical variables are expressed as the mean ± standard deviation for parametric tests or median (interquartile range) for nonparametric tests, and categorical variables are presented as percentages.
The severity of disease was considered based on the Centers for Disease Control and Prevention criteria: (1) dyspnea, (2) respiratory frequency ≥ 30/minute, (3) blood oxygen saturation < 93%, and/or (4) lung infiltrates > 50% of the lung field within 24 to 48 hours.
At the time of admission (baseline), the severity of COVID-19 was observed similar in both groups (67.9% in the placebo group and 60.4% in the 25(OH)D3 group, P = .41). In terms of prognostic factors of COVID-19, there were no significant differences between the 2 groups (Table 2 ).
Table 2.
Prognostic Factors for Coronavirus Disease 2019 at Baseline
| Baseline characteristics | N (25[OH]D3/placebo) | 25(OH)D3 | Placebo | P value |
|---|---|---|---|---|
| Age ≥ 65 years | 53/53 | 13% (7) | 13% (7) | 1.0 |
| Disease severity (based on the CDC criteria) | 53/53 | 68% (36) | 60% (32) | .4 |
| History of chronic disorders | ||||
| Hypertension | 53/53 | 34% (18) | 28% (15) | .5 |
| Cardiac disorder | 53/53 | 9% (5) | 15% (8) | .4 |
| Diabetes mellitus | 53/53 | 26% (14) | 21% (11) | .5 |
| Immunologic | 53/53 | 4% (2) | 0 | .5a |
| Liver | 53/53 | 1.9% (1) | 0 | 1.0a |
| Renal | 53/53 | 4% (2) | 2% (1) | 1.0a |
| Neurologic | 53/53 | 4% (2) | 0% | .5a |
| Lung | 53/53 | 7.5% (4) | 13% (7) | .3 |
| Lymphocytes < 800 | 52/53 | 19% (10) | 22% (12) | .6 |
Abbreviations: CDC = Centers for Disease Control and Prevention; 25(OH)D3 = 25-hydroxyvitamin D3.
At baseline, there was no significant difference in the history of chronic disorders including hypertension; cardiovascular disorder; diabetes mellitus; lung, liver, and kidney diseases; and neurologic and immunologic disorders.
Fisher exact test.
Improvement of Circulating Serum Concentrations of 25(OH)D3
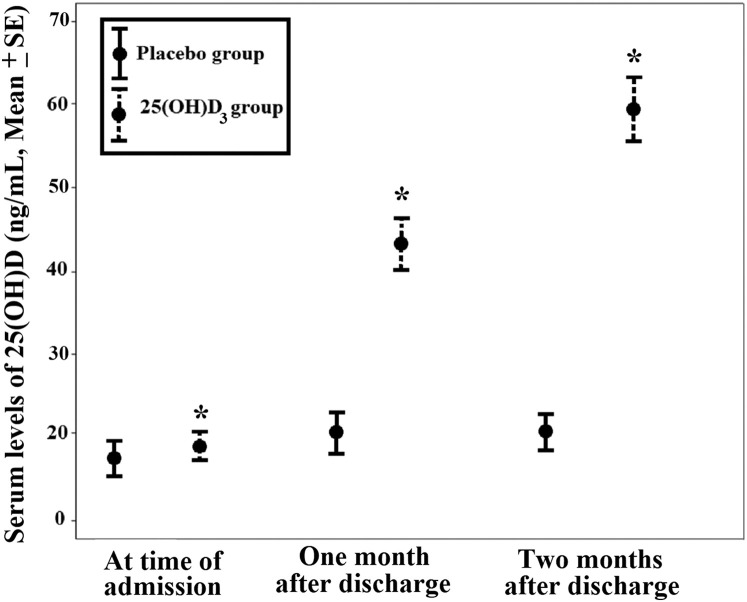
Figure 2 shows the 25(OH)D3 concentrations at baseline and after the first and second months of follow-up. After 30 days of using 25(OH)D3 or placebo, the circulating 25(OH)D3 concentrations significantly increased in patients who received 25(OH)D3 compared with those in the placebo group (treatment group, 42.0 ± 13.7 ng/mL, vs. placebo group, 19.3 ± 8.5 ng/mL) (Fig. 2). The delta serum 25(OH)D3 concentration was 23.6 ± 10.4 ng/mL in the treatment group compared with 0.8 ± 4.2 ng/mL in the placebo group; 79.4% of patients in the treatment group and 12.5% of patients in the placebo group had circulating 25(OH)D3 concentrations of >30 ng/mL.
Fig. 2.
Alterations in the serum 25-hydroxyvitamin D3 (25[OH]D3) concentrations in the 25(OH)D3 and placebo groups. The serum 25(OH)D3 concentrations significantly increased in patients who received 25(OH)D3 compared with those in the placebo group. After 30 days of ingesting 25(OH)D3 or placebo, the circulating 25(OH)D3 concentrations significantly increased in patients who received 25(OH)D3 (N = 34) compared with those in the placebo group (N = 24). Treatment group, 42.0 ± 2.3 ng/mL, versus placebo, 19.3 ± 1.7 ng/mL. After 60 days, 24 patients in the treatment group had a serum 25(OH)D3 concentration of 59.6 ± 3.8 ng/mL compared with 19 patients in the placebo group who had a serum 25(OH)D3 concentration of 19.4 ± 1.6 ng/mL. The error bars are mean ± standard error; ∗P < .001.
After 60 days of using oral 25(OH)D3 or placebo, the circulating 25(OH)D3 concentrations significantly increased in patients who received 25(OH)D3 compared with those in the placebo group (treatment group, 59.6 ± 18.6 ng/mL, vs. placebo group, 19.4 ± 7.0 ng/mL). The delta serum 25(OH)D3 concentration was 40.02 ± 19.2 ng/mL in the treatment group compared with 1.4 ± 6.5 ng/mL in the placebo group; all patients in the treatment group and 10.5% of patients in the placebo group had circulating 25(OH)D3 concentrations of >30 ng/mL.
SMD was used to express the size of the intervention effect on increasing the circulating vitamin D concentrations in the treatment group compared with the placebo group. The treatment group had 1.92 effect size (SMD , 1.92; 95% confidence interval [CI], 1.38-2.45) on increased circulating 25(OH)D3 concentrations after the first month of follow-up and 2.73 effect size (SMD, 2.73; 95% CI, 2.11-3.35) on increased circulating 25(OH)D3 concentrations after the second month of follow-up.
The compliance of taking capsules (25[OH]D3 or placebo) was 89% in the treatment group (95% CI, 85-94) versus 93% in the placebo group (95% CI, 90-96).
COVID-19 Clinical Features
There was an overall trend for lower hospitalization duration in the 25(OH)D3 group compared with that in the placebo group, which was not statistically significant (median [IQR], 5 [3] vs. 6 [5.5]; P = .1).
Among the patients in the treatment group, 6 were admitted in an intensive care unit (ICU) compared with 10 in the placebo group. In addition, 2 patients in the treatment group and 5 patients in the placebo group needed a ventilator. Death occurred in 3 patients in the treatment group compared with the 5 patients in the placebo group. There were no statistically significant differences in ICU admissions, need for ventilation, and rate of death between patients who received 25(OH)D3 and those who received the placebo (Table 3 ).
Table 3.
Clinical and Biochemical Outcomes
| Clinical outcomes | N (25[OH]D3/placebo) | 25(OH)D3 | Placebo | P value |
|---|---|---|---|---|
| Hospitalization day | 53/53 | 5 (3) | 6 (5.5) | .1 |
| Death | 53/53 | 6% (3) | 9% (5) | .7a |
| Oxygen therapy | 53/53 | 60% (32) | 64% (34) | .7 |
| Intubation | 53/53 | 4% (2) | 9% (5) | .4a |
| Ventilator | 53/53 | 4% (2) | 9% (5) | .4a |
| ICU admitted | 53/53 | 11% (6) | 19% (10) | .3 |
| ICU (days) (range) | 53/53 | 7 (0-7) | 11 (0-11) | .2 |
| Biochemical outcome | ||||
| Mean difference :WBC (×103) | 53/52 | 0.1 ± 3.1 | 1.8 ± 4.2 | .02 |
| Mean difference: lymphocyte (×103) | 47/51 | 2.8 ± 12.3 | −2.7 ± 11.9 | .02 |
| Mean difference: LDH (U/L) | 27/18 | −5 (177) | −41.5 (221) | .6 |
| Mean difference: neutrophil (×103) | 47/51 | −0.2 (0.8) | 0.23 (1.1) | .01 |
| Mean difference: platelet (×103) | 53/50 | 29 (83.5) | 21 (65) | .6 |
| NLR at the date of baseline | 52/52 | 4.2 ± 3.8 | 3.4 ± 1.6 | .3 |
| NLR at the date of release | 48/52 | 3.3 ± 2.5 | 5.3 ± 4.8 | .02 |
| Treatments | ||||
| Antiviral therapy | 53/53 | 4% (2) | 5% (3) | 1 |
| Corticosteroid therapy | 53/53 | 40% (21) | 53% (28) | .24 |
Abbreviations: ICU = intensive care unit; LDH = lactate dehydrogenase; NLR = neutrophil-to -lymphocyte ratio; 25(OH)D3 = 25-hydroxyvitamin D3; WBC = white blood cell.
Values in bold indicate statistical significance (P < .05).
Fisher exact test.
During hospitalization, all patients were treated with hydroxychloroquine and antibiotics (azithromycin or ceftriaxone). There was no significant difference between the groups in the proportion of patients treated with corticosteroids (<10 mg/day of dexamethasone or ≤25 mg of methylprednisolone) or antiviral drugs (interferon). In addition, in the regression model, antiviral or glucocorticoid treatment had no significant effect on the NLR and no effect on the relationship between 25(OH)D3 treatment and decreasing the NLR. The biochemical tests at release time are presented in Supplementary Table 2. To consider the effect of the oral consumption of 25(OH)D3 during hospitalization, the mean differences of all biochemical and hematologic tests were calculated; at baseline and at the time that they were discharged from the hospital.
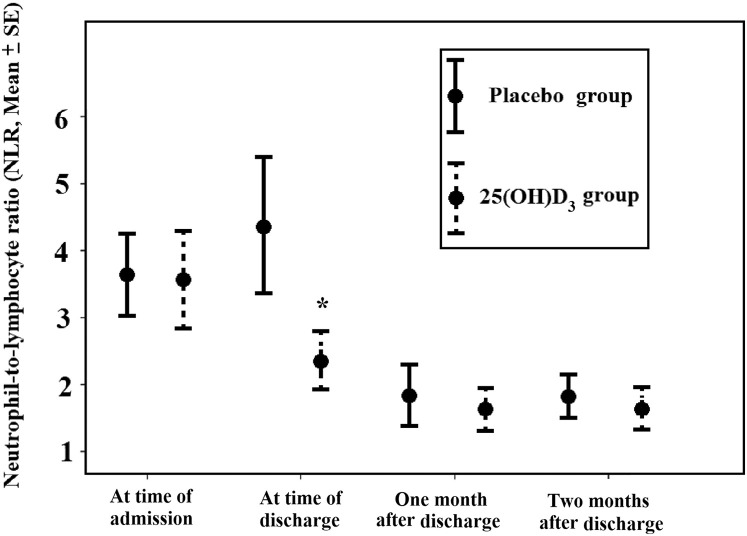
Patients who received 25(OH)D3 had a significant increase in the percentage of lymphocytes (P = .03). In the 25(OH)D3 group, the NLR was less than that in the placebo group (SMD, −0.81; 95% CI, −1.21 to −0.41). After the patients were discharged from the hospital, they had a follow-up visit 1 and 2 months later at which time the NLR in their circulation was determined. The circulating NLR decreased in both groups and were not statistically significantly different (Fig. 3 ).
Fig. 3.
The neutrophil-to-lymphocyte ratio (NLR) in the 25-hydroxyvitamin D3 (25[OH]D3) and placebo groups at the time of hospitalization and after release date. The NLR at the time of discharge significantly decreased in patients who received 25(OH)D3 compared with that in the placebo group. After the patients were discharged from the hospital, they had a follow-up visit 1 and 2 months later at which time the NLR in their circulation was determined. The circulating NLR decreased in both groups and were no statistically significantly different. The NLRs at the first month of follow-up in patients who received 25(OH)D3 (n = 33) and placebo (n = 28) were 1.7 ± 0.2 and 1.8 ± 0.2, respectively. After 2 months of follow-up, the NLRs in the treatment group (n = 32) and placebo group (n = 22) were 1.9 ± 0.3 and 1.9 ± 0.2, respectively. The error bars are mean ± standard error; ∗P < .05.
There was no significant difference in the mean changes in the platelet number and serum concentrations of LDH between the 2 groups (P = .96 and P = .57, respectively).
The data analysis showed that there were no significant differences in readmission. There was no mortality in the 2 groups after the first and second months of follow-up.
Regarding the modifier role of 25(OH)D3 on treatment, the relationship of NLR was considered with the main outcomes of COVID-19 including ICU admission days and mortality.
There was a significant correlation between lower NLR and reduced ICU admission days (rho = 0.3; P = .004). In a logistic regression model, after adjusting for age, sex, body mass index, and history of chronic disorders, there was an independent association between the NLR at the time of discharge and needing ICU admission (P = ..002; odds ratio (OR), 1.2).
Although in our study population a few patients died, the NLR at the last day of hospitalization was approximately 4 times higher than that of patients who survived (median [IQR)], 15.7 [7.7] vs. 3.1[5.1]).
Among biochemical outcomes, there was a significant correlation between the NLR and LDH (P = .02; r = 0.3). However, after adjusting for age, sex, and history of chronic disorders, there was no significant association between the NLR and LDH at the time of discharge (P = .08).
Discussion
To assess the therapeutic effect of rapidly improving vitamin D status in hospitalized patients with COVID-19, we designed a randomized, double-blinded, placebo-controlled clinical trial whereby hospitalized patients received either 25 μg of 25(OH)D3 (calcifediol) or placebo daily for 60 days.
In our trial, the rationale was to administer 25(OH)D3 to the patients with COVID-19 to increase and sustain the circulating serum 25(OH)D3 concentrations and relate this effect to clinical outcomes. One of the advantages of using 25(OH)D3 is that the serum 25(OH)D3 concentration increases more rapidly than in using vitamin D3 since conversion to 25(OH)D3 is not required.21 Therefore, oral 25(OH)D3 is able to correct vitamin D deficiency more rapidly and consistently than oral vitamin D3.21 , 22
Based on vitamin D biologic potency, 1 IU of vitamin D3 is equivalent to 0.025 μg; proportionally, 25 μg = 1000 IU of vitamin D3.23 However, there is no gold standard for the equivalence between IUs and molecular mass with 25(OH)D3.
Clinical trials have demonstrated that oral 25(OH)D3 was 3 to 6 times more effective in rapidly increasing the circulating 25(OH)D3 concentrations compared with oral vitamin D3 on a weight basis.22 Therefore, 25 μg of 25(OH)D3 is not equivalent to 25 μg (1000 IU) of vitamin D3. It would be equivalent to 75 to 150 μg or 3000 to 6000 IU of vitamin D3. Barger-Lux et al24 evaluated different dosages of oral 25(OH)D3 (calcifediol) compared with vitamin D3. They showed that “when using dosages ≤ 25 μg/day, serum 25OHD increased by 1.5 ± 0.9 nmol/L for each 1 μg of vitamin D3, whereas this was 4.8 ± 1.2 nmol/L for oral 25(OH)D3 and the relative potency of 25(OH)D3 to vitamin D3 was 3 times higher. Also the authors stated the highest dose of 25(OH)D3 (50 μg/day), was 7–8-fold more potent than vitamin D3 with similar dosages.”
Charoenngam et al25 in a randomized, placebo-controlled crossover study investigated the pharmacokinetics of oral 25(OH)D3 and oral vitamin D3 in healthy and obese adults and in adult patients with fat malabsorption syndromes: a 900-μg single dose of either vitamin D3 or 25(OH)D3. They observed that the blood 25(OH)D3 concentrations rapidly increased and reached a peak concentration within 8 hours, whereas when the same healthy adult ingested this same amount of vitamin D3, the serum concentrations of 25(OH)D3 gradually increased and reached a maximum blood concentration within 48 to 72 hours.25 They also observed that patients with obesity and fat malabsorption who were unable to increase their circulating 25(OH)D3 concentrations to a similar degree as healthy adults after ingesting vitamin D3 were able to increase their circulating 25(OH)D3 concentrations to the same degree after ingesting 25(OH)D3 compared with the healthy adults.25
In the present trial, the mean baseline serum 25(OH)D3 concentration of all participates was at the range of 2 to 29 ng/mL. Our findings indicated that the size of the intervention effect on increasing the circulating 25(OH)D3 concentrations in the treatment group was significantly higher than in the placebo group. Using 25 μg of 25(OH)D3 daily in the treatment group had effect sizes of 1.92 and 2.73 on increased circulating 25(OH)D3 concentrations compared with the placebo group after the first and second months of follow-up, respectively. Our observation is consistent with the conclusion made by Quesada-Gomez and Bouillon14 who evaluated 9 randomized controlled trials (RCTs) and concluded that “the conversion efficacy of oral 25(OH)D3 would be 3.2-fold more effective when compared with the same dosages of oral vitamin D3 and also, oral calcifediol is a linear dose-response curve, irrespective of baseline serum 25OHD, whereas the rise in serum 25OHD is lower after oral cholecalciferol, when baseline serum 25OHD is higher.”
As organ damage from the cytokine storm and proliferation of the SARS-CoV-2 progress rapidly soon after the infection and once the damage is done, it is difficult to reverse. Thus, the more rapid increase in the serum 25(OH)D3 concentrations may provide an advantage in reducing morbidity and mortality associated with infectious diseases like COVID-19.
Increasing the total and free 25(OH)D3 concentrations could result in rapid entry into its target innate and adaptive immune cells, resulting in the production of 1,25(OH)2D, which interacts with the vitamin D receptor to modulate immune function).16 , 18 , 26 Therefore, using a high dose of 25(OH)D3 at the time of hospital admission may help in the treatment of COVID-19 by preventing the cytokine storm and subsequent acute respiratory distress syndrome, which is commonly the cause of mortality.
Our data showed that patients who received oral 25(OH)D3 demonstrated a statistically significantly lower NLR with a −0.81 efficacy (compared with those in the placebo group). During an inflammatory response, leukocytes act as an innate immune response, and lymphocytes are responsible for the specificity of adaptive immune response. They circulate in the blood and central lymphoid tissues and participate in a variety of host defense mechanisms against viral infections. These include “cell-mediated reactions against infected cells and particularly those involving cytotoxic T lymphocytes, co-operation in the induction of antibody responses, and the production of immune interferon.”27 Recent observational studies in patients with COVID-19 revealed that most of the infected patients have higher leukocyte and lower lymphocyte counts.28 The NLR is also considered an inflammatory marker and a prognostic factor of systemic inflammation that is increased in patients with COVID-19 with severe clinical consequences.29 In a recent meta-analysis by Lagunas-Rangel,30 the NLR values were found to increase in patients with severe COVID-19 with a 2.4 efficacy. Consistent with these studies, our data showed the NLR at the time of enrollment was a significant predictor of needing intensive care. Although only a few patients died in our study, those who died had an NLR that was 4 times higher than the NLR at the time the patients were discharged from the hospital.
Based on these findings, the 25(OH)D3 intervention was significantly associated with decreased NLR in the hospitalized patients with COVID-19 compared with the hospitalized patients who received the placebo. Although the decrease in the circulating NLR was associated with improved clinical outcomes, we could not conclude that the decrease in NLR was solely the effect of 25(OH)D3 treatment and, therefore, that the increasing 25(OH)D3 concentration was responsible for the change in clinical outcomes. Some studies have reported that vitamin D deficiency is associated with high mortality and morbidity in patients with COVID-19. A recent meta-analysis on 27 observational studies identified a positive association between vitamin D deficiency and the severity of the COVID-19 (OR, 1.64; 95% CI, 1.30-2.09). They also revealed that vitamin D insufficiency increases hospitalization (OR, 1.81; 95% CI, 1.41-2.21) and mortality from COVID-19 (OR, 1.82; 95% CI, 1.06-2.58).7
In a clinical trial by Entrenas Castillo et al,9 patients with COVID-19 who received a high dose of oral 25(OH)D3 had a reduced need for ICU admission. These patients received 532 μg of oral 25(OH)D3 on the first day of admission and 266 μg of oral 25(OH)D3 on days 3 and 7 of hospitalization and then weekly until discharge or ICU admission.9 Similar to our study, 2 retrospective cohort studies31 , 32 on patients admitted for COVID-19 reported that treatment with calcifediol reduced the risk of requirement for critical care by more than 80% and reduced the mortality risk by more than 70%.”31
In another clinical trial, Annweiler et al33 considered the efficacy of a bolus dose of 80 000 IU of vitamin D3 supplementation taken during COVID-19 or in the preceding month in frail elderly. The survival rate was twofold higher compared with that in the control group, with an adjusted hazard ratio of 0.11 (95% CI, 0.03-0.48). They reported that “bolus vitamin D3 supplementation during COVID-19 or in the preceding month was associated with less severe COVID-19 and better survival rate in elderly.”
Our data are consistent with these observations, where we observed an overall lower trend for hospitalization and ICU duration, need for ventilator assistance, and mortality in the 25(OH)D3 group compared with that in the placebo group. We could not show a significant improvement in the clinical outcomes in patients who ingested 25(OH)D3 compared with that in the control group, this could be due to the need to more rapidly improved serum 25(OH)D3 concentrations by administering a higher dose of 25(OH)D3 as has been recently reported.9 , 33 , 34
In our patient population, 50% of participants had at least a history of 1 chronic disorder. Based on epidemiology studies, approximately half of all patients suffering from COVID-19 had a history of chronic disorders like hypertension, diabetes, cardiovascular disease, kidney disorder, cancers, and immunologic diseases, and the mortality rate increased in the presence of serious comorbid medical conditions.34 , 35 Based on various RCTs and meta-analyses, it has been observed that vitamin D supplementation, especially in vitamin D–deficient patients, can provide a clinically beneficial effect in some of these medical conditions. However, to treat COVID-19 in hospitalized patients, the rapid increase in the circulating serum 25(OH)D3 concentrations may help reduce the risk of morbidity and mortality in these patients with a history of other chronic disorders.36
Of note, up to now, there is no standardized practice and/or cost benefit of using oral 25(OH)D3 compared with oral vitamin D3.14 Based on the market prices in Italy in 2019, the cost per IU of 25(OH)D3 could be approximately 6 times lower than that of cholecalciferol.17 Based on these findings, we strongly recommend further RCTs to consider 25(OH)D3 as an alternative vitamin D supplementation in vitamin D–deficient or vitamin D–insufficient adults with an acute medical condition like COVID-19.
The major strengths of this study include its experimental design, which was randomized, double-blinded, and placebo-controlled, and a high adherence rate. However, this study also has several weaknesses and limitations. This was a pilot study that evaluated biochemical and clinical outcomes in patients with COVID-19 who received 25 μg of 25(OH)D3 daily orally. This dose, which is equivalent to ingesting 3000 to 6000 IU/day of a vitamin D3 supplement, would be considered to be two- to threefold higher than what is recommended by the Endocrine Society guidelines on vitamin D for adults, which is 1500 to 2000 IU daily.13 The analysis of this pilot study showed that this dose of 25(OH)D3 only had effect on decreasing the NLR that has been related to improved clinical outcomes.29 , 30 Although we did not observe that the significant decrease in the NLR in the patients who ingested 25(OH)D3 was related to improved clinical outcomes compared with that in the control group, this could be due to the need to more rapidly improve serum 25(OH)D3 concentrations by administering a higher dose of 25(OH)D3, as has been recently reported.9 , 33 , 37 This pilot study was performed on 53 patients in each group. It was underpowered for detecting significant differences in clinical outcome measures. RCTs with a larger sample size and higher dose of 25(OH)D3 are needed to confirm the potential effect of 25(OH)D3 on reducing clinical outcomes in patients with COVID-19.
Conclusions
Our findings indicate that using 25 μg of 25(OH)D3 daily orally is safe and effective in increasing and maintaining optimal 25(OH)D3 serum concentrations in adults with COVID-19. Treatment with oral 25(OH)D3 has a potential benefit in improving immune function by increasing the lymphocyte percentage and decreasing the RCT in hospitalized patients with COVID-19. Indeed, our findings showed that the 25(OH)D3 intervention significantly decreased the NLR in patients with COVID-19 that was associated with improved clinical outcomes.
The ability to rapidly increase and sustain circulating 25(OH)D3 concentrations will result in the immediate availability of 25(OH)D3 that can be quickly converted to the immunomodulatory hormone 1,25(OH)2D. Therefore, there is a strong rationale to consider using 25(OH)D3 to improve the patient’s vitamin D status to help maximize their immune system and fight the COVID-19 pandemic.
Ethics and Dissemination
The study protocol was approved by the Ethics Committee of Tehran University of Medical Sciences (approval number: IR.TUMS.VCR.REC.1399.061). Dissemination plans include academic publications, conference presentations, and social media.
Trial Registration
The protocol was registered with the Iranian Registry of Clinical Trials (IRCT) on April 11, 2020 (IRCT20200401046909N1) and U.S. National Institutes of Health (NCT04386850) on May 11, 2020.
Acknowledgment
The authors thank all health providers and laboratory staff of Ziaeian Hospital, Shariati Hospital, and Shohada Pakdasht Hospital. Dishman Carbogen Amcis Ltd, as a partner of Boston University, manufactured and supplied both the active form from its subsidiary location in the Netherlands and the finished dosage form from its facility in Ahmedabad, India for the trials. Arpit J. Vyas, the Global Managing Director of Dishman Carbogen Amcis Ltd commented “We are proud to have been able to support this investigation that could facilitate the global fight against COVID-19. Our collaboration with BU and Dr. Holick continues to demonstrate the remarkable properties of Vitamin D analogs and their potential role in treating many unmet clinical needs.” This work was supported by Tehran University of Medical Sciences (grant no.: 47095-235-1-99).
Disclosure
M.F.H. has been a consultant for Quest Diagnostics Inc, Ontometrics Inc, and Biogena Inc, received a grant from Carbogen Amcis BV, and has been on the speaker’s bureau for Abbott Inc. The other authors have no multiplicity of interest to disclose.
Supplementary Material
References
- 1.COVID 19 information. Accessed Month DD, YYYY. https://covid19.continualflight.com/
- 2.Lippi G., Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Liang W.H., Zhao Y., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martineau A.R., Jolliffe D.A., Hooper R.L., et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maghbooli Z., Sahraian M.A., Ebrahimi M., et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PloS One. 2020;15(9) doi: 10.1371/journal.pone.0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Kaufman H.W., Niles J.K., Kroll M.H., Bi C., Holick M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PloS One. 2020;15(9) doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira M., Dantas Damascena A., Galvão Azevedo L.M., de Almeida Oliveira T., da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020:1–9. doi: 10.1080/10408398.2020.1841090. [DOI] [PubMed] [Google Scholar]
- 8.Murai I.H., Fernandes A.L., Sales L.P., et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325(11):1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Entrenas Castillo M., Entrenas Costa L.M., Vaquero Barrios J.M., et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant W.B., Lahore H., McDonnell S.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goncalves-Mendes N., Talvas J., Dualé C., et al. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front Immunol. 2019;10:65. doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misra D.P., Agarwal V., Gasparyan A.Y., Zimba O. Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol. 2020;39(7):2055–2062. doi: 10.1007/s10067-020-05073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 14.Quesada-Gomez J.M., Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos Int. 2018;29(8):1697–1711. doi: 10.1007/s00198-018-4520-y. [DOI] [PubMed] [Google Scholar]
- 15.Biancuzzo R.M., Clarke N., Reitz R.E., Travison T.G., Holick M.F. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab. 2013;98(3):973–979. doi: 10.1210/jc.2012-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischoff-Ferrari H.A., Dawson-Hughes B., Stöcklin E., et al. Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res. 2012;27(1):160–169. doi: 10.1002/jbmr.551. [DOI] [PubMed] [Google Scholar]
- 17.Cesareo R., Falchetti A., Attanasio R., Tabacco G., Naciu A.M., Palermo A. Hypovitaminosis D: is it time to consider the use of calcifediol? Nutrients. 2019;11(5):1016. doi: 10.3390/nu11051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouillon R., Marcocci C., Carmeliet G., et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. 2019;40(4):1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hossein-Nezhad A., Holick M.F. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88(7):720–755. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maghbooli Z., Omidifar A., Varzandi T., Salehnezhad T., Sahraian M.A. Reduction in circulating vitamin D binding protein in patients with multiple sclerosis. BMC Neurol. 2021;21(1):168. doi: 10.1186/s12883-021-02200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charoenngam N., Holick M.F. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12(7):2097. doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sosa Henríquez M., Gómez de Tejada Romero M.J. Cholecalciferol or calcifediol in the management of vitamin D deficiency. Nutrients. 2020;12(6):1617. doi: 10.3390/nu12061617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro-Valverde C., Sosa-Henríquez M., Alhambra-Expósito M.R., Quesada-Gómez J.M. Vitamin D3 and calcidiol are not equipotent. J Steroid Biochem Mol Biol. 2016;164:205–208. doi: 10.1016/j.jsbmb.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Barger-Lux M.J., Heaney R.P., Dowell S., Chen T.C., Holick M.F. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8(3):222–230. doi: 10.1007/s001980050058. [DOI] [PubMed] [Google Scholar]
- 25.Charoenngam N., Kalajian T.A., Shirvani A., et al. A pilot-randomized, double-blind crossover trial to evaluate the pharmacokinetics of orally administered 25-hydroxyvitamin D3 and vitamin D3 in healthy adults with differing BMI and in adults with intestinal malabsorption. Am J Clin Nutr. 2021;114(3):1189–1199. doi: 10.1093/ajcn/nqab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39(2):365–379. doi: 10.1016/j.ecl.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denman A.M. Lymphocyte function and virus infections. J Clin Pathol Suppl (R Coll Pathol) 1979;13:39–47. doi: 10.1136/jcp.s3-13.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang W., Berube J., McNamara M., et al. Lymphocyte subset counts in COVID-19 patients: a meta-analysis. Cytometry A. 2020;97(8):772–776. doi: 10.1002/cyto.a.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borges L., Pithon-Curi T.C., Curi R., Hatanaka E. COVID-19 and neutrophils: the relationship between hyperinflammation and neutrophil extracellular traps. Mediators Inflamm. 2020;2020:8829674. doi: 10.1155/2020/8829674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020;92(10):1733–1734. doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcala-Diaz J.F., Limia-Perez L., Gomez-Huelgas R., et al. Calcifediol treatment and hospital mortality due to COVID-19: a cohort study. Nutrients. 2021;13(6):1760. doi: 10.3390/nu13061760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogues X., Ovejero D., Pineda-Moncusí M., et al. Calcifediol treatment and COVID-19-related outcomes. J Clin Endocrinol Metab. 2021;106(10):e4017–e4027. doi: 10.1210/clinem/dgab405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annweiler C., Hanotte B., Grandin de l'Eprevier C., Sabatier J.M., Lafaie L., Célarier T. Vitamin D and survival in COVID-19 patients: a quasi-experimental study. J Steroid Biochem Mol Biol. 2020;204:105771. doi: 10.1016/j.jsbmb.2020.105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 36.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCullough P.J., Lehrer D.S., Amend J. Daily oral dosing of vitamin D3 using 5000 to 50,000 international units a day in long-term hospitalized patients: insights from a seven year experience. J Steroid Biochem Mol Biol. 2019;189:228–239. doi: 10.1016/j.jsbmb.2018.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.