Figure 1. Study Flowchart.
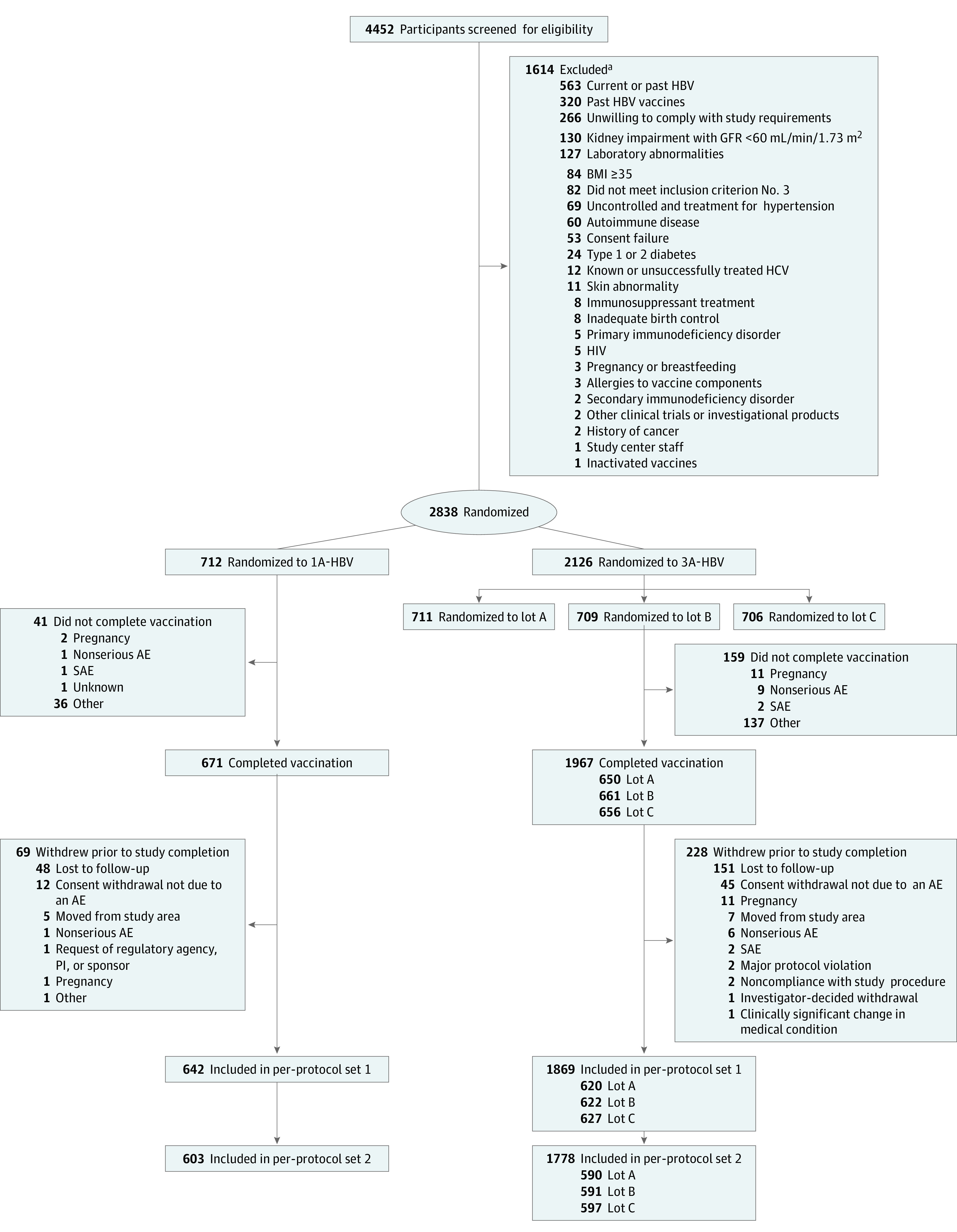
Per-protocol set 1 included those who received all 3 vaccinations, had evaluable serum immunogenicity samples at baseline and at the point of interest, were seronegative at baseline, and had no major protocol deviations leading to exclusion. Per-protocol set 2 included those in per-protocol set 1, except those who attended study visits 3 and 4 outside of the defined windows. 1A-HBV indicates single-antigen hepatitis B virus vaccine; 3A-HBV, 3-antigen HBV; AE, adverse event; and SAE, serious AE.
aIndividuals may have multiple reasons for exclusion.
