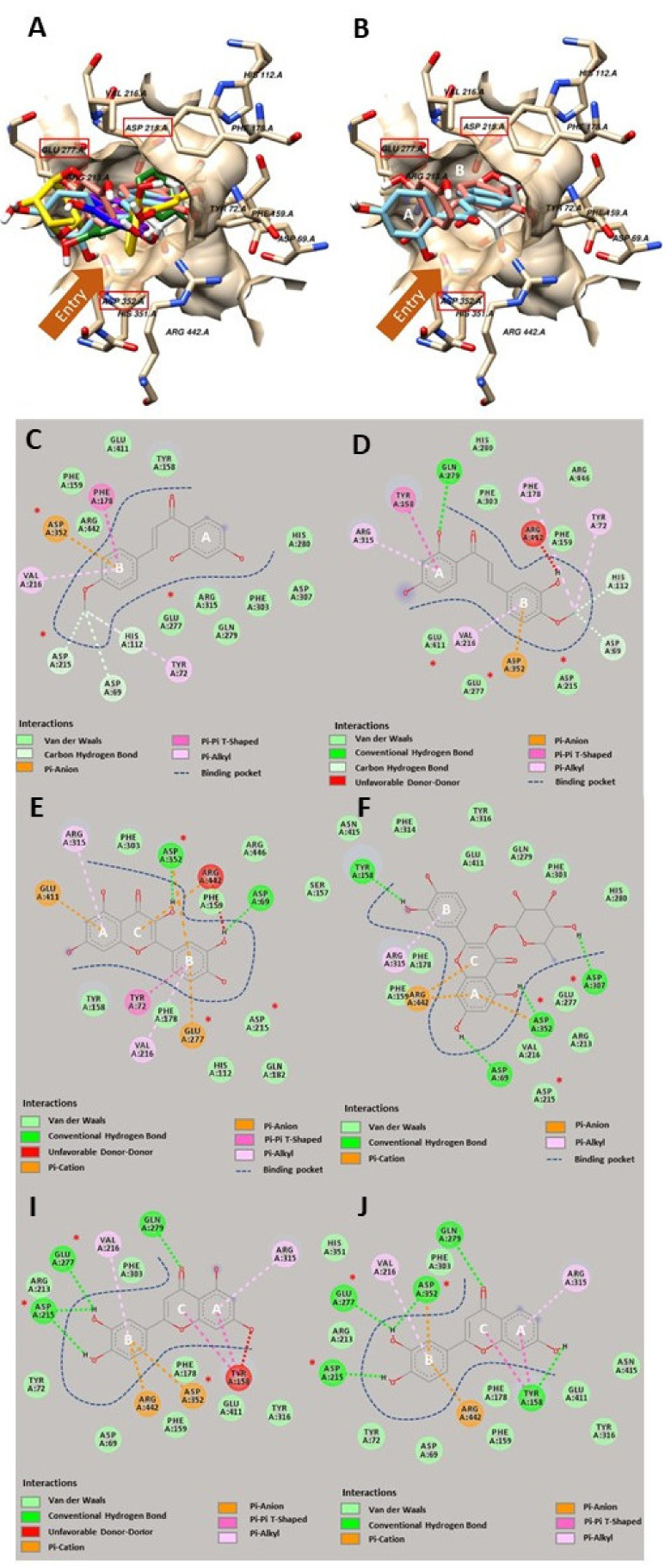
Figure 2.
The molecular interaction between amino acids in the active site of glucosidase (Ivory) and glucose as a native ligand (White) and isolated flavonoids, luteolin (Blue), 5-deoxyluteolin (Pink), quercetin (Green), quercitrin (Yellow), chalcones, 3,2’,4’-trihydroxy-4-methoxychalcone (Red) and 4-methyl ether isoliquiritigenin (Cyan) from Bauhinia pulla (A). Structural alignment of 3,2’,4’-trihydroxy-4-methoxychalcone (Red) and 4-methyl ether isoliquiritigenin (Cyan) in the active site of glucosidase (Ivory) (B). 2D interaction diagram of 4-methyl ether isoliquiritigenin-glucosidase complex (C), 3,2’,4’-trihydroxy-4-methoxychalcone-glucosidase complex (D), quercetin-glucosidase complex (E), quercitrin-glucosidase complex (F), luteolin-glucosidase complex (I), and 5-deoxyluteolin-glucosidase complex (J). Catalytic residues in the active site indicated by red boxes and asterisks. A, B and C indicate the ring system in both flavonoids and chalcones.