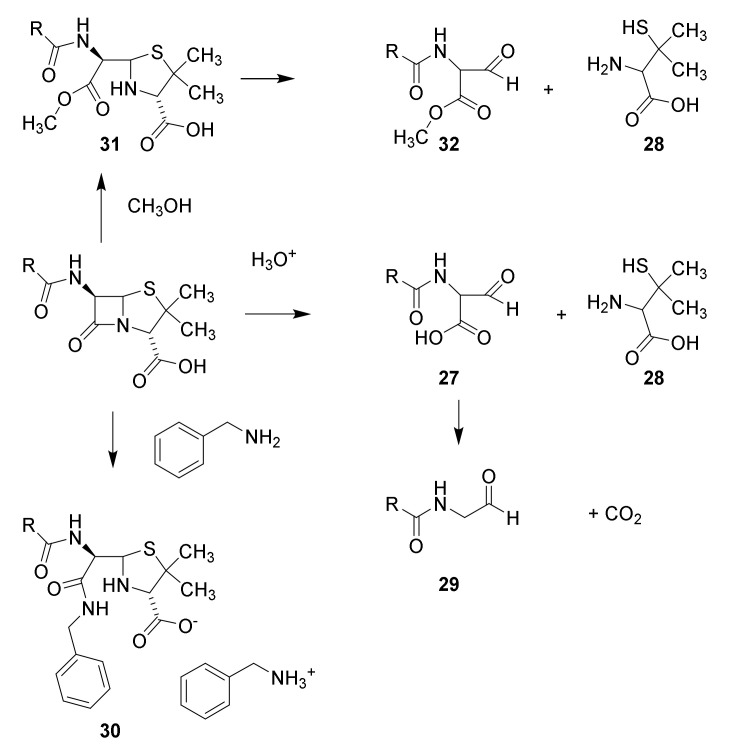
Scheme 2.
Structure elucidation of penicillin. Hydrolysis with mineral acid affords carbon dioxide, penicillamine (28) and penaldic acid (27), which is labile and spontaneously converted into penillo-aldehyde (29). Treatment with benzylamine yields aminolysis of the β-lactam to give the benzylammonium salt of the benzylamide 30. Methanolysis affords the monomethyl ester of methyl penicilloate (31), which may be converted into methyl penaldoate and (32) and penicillamine (28). The suggested structures have been confirmed by syntheses [47,48].