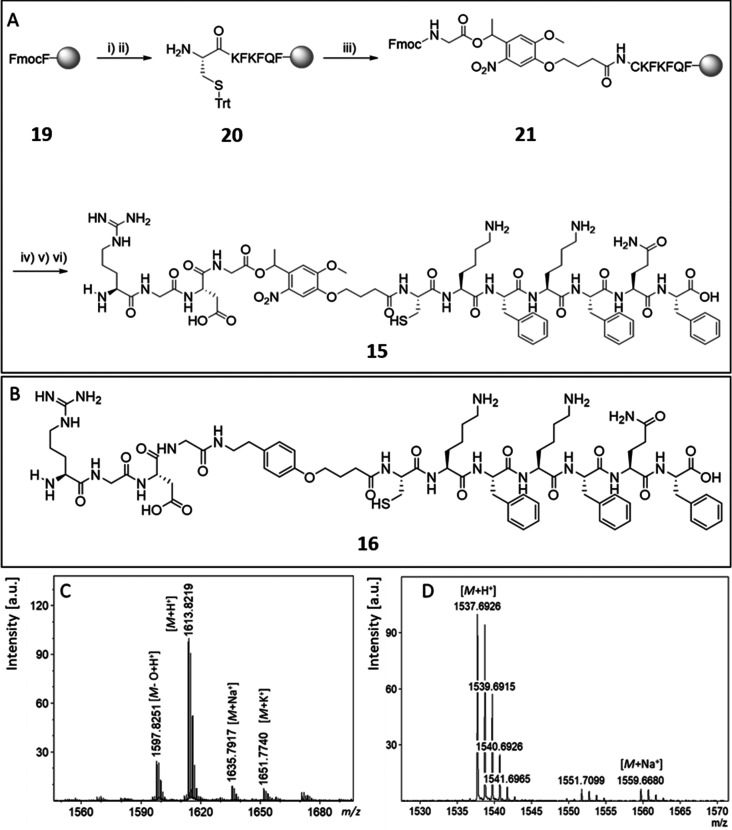
Figure 1.
(A) Microwave assisted solid-phase peptide synthesis of the photocleavable peptide RGD-PCL-CKFKFQF (15): (i) Fmoc deprotection; (ii) coupling of Fmoc-Gln(Trt)-OH/Fmoc-Phe-OH/Fmoc-Lys(Boc)-OH/Fmoc-Cys(Trt)-OH; (iii) coupling of PCL; (iv) coupling of Fmoc-Asp(OtBu)-OH/Fmoc-Gly-OH/Fmoc-Arg(Pbf)-OH; (v) Fmoc deprotection; (vi) resin cleavage. (B) Nonphotocleavable peptide RGD-NCL-CKFKFQF (16). (C) MALDI-ToF-MS spectra of the purified peptide (15) confirming successful synthesis: calcd for [M + H]+, 1613.74 g/mol; found 1613.82 m/z, calcd for [M + Na]+, 1635.72 g/mol; found 1635.79 m/z., calcd for [M+K]+, 1651.694 g/mol found 1651.77 m/z. (D) MALDI-ToF-MS spectra of the purified peptide (16): calcd for [M + H]+, 1537.76 g/mol; found 1537.69 m/z., calcd for [M + Na]+, 1559.74 g/mol; found 1559.66 m/z.