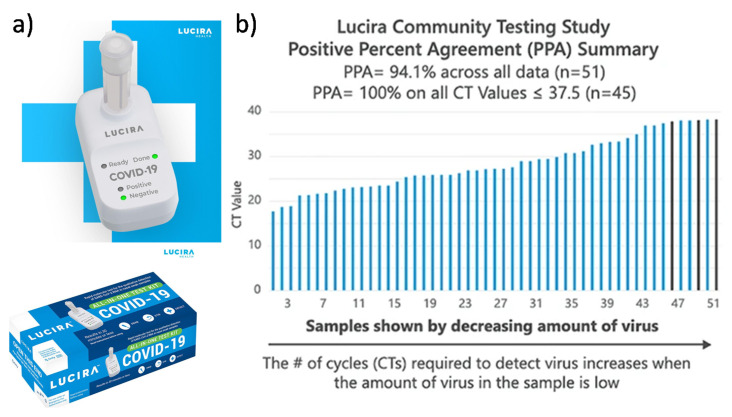
Figure 5.
Lucira’s COVID-19 All-In-One Test Kit product and community testing data. (a) The single-use, over-the-counter test kit intended for the molecular detection of SARS-CoV-2 RNA from nasal swab samples. (b) In a Community Testing Study, the Lucira test was compared with high sensitivity Hologic Panther Fusion SARS-CoV-2 test, and achieved a 94% positive percent agreement (PPA) and a 98% negative percent agreement (NPA). Images are reproduced and adapted with permission from Lucira Health [25].