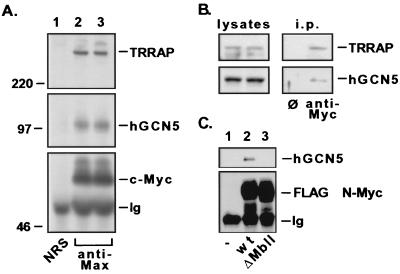
FIG. 3.
Myc family oncoproteins recruit hGCN5 in human cells. (A) 293 cells were lysed under nondenaturing conditions, and c-Myc–Max dimers were immunoprecipitated with antisera against Max (lanes 2 and 3). A parallel precipitation was performed with nonimmune rabbit serum (NRS) as a control (lane 1). Precipitated proteins were resolved by SDS-PAGE (8% gel) and subjected to immunoblotting for either c-Myc (lower panel), hGCN5 (middle panel), or TRRAP (upper panel). Numbers at the left (in kilodaltons) indicate positions of size markers. The position of the heavy-chain polypeptide from the precipitating antibody is indicated (Ig). (B) 293 cells were lysed and subjected to immunoprecipitation with either a control monoclonal antibody (ø) or a monoclonal antibody directed against c-Myc. Immunoprecipitates (i.p.) were blotted and probed for TRRAP (upper panel) and hGCN5 (lower panel). (C) 293 cells were transiently transfected with a cytomegalovirus-driven expression vector encoding FLAG epitope-tagged versions of either wild-type (wt) murine N-Myc (lane 2) or a mutant lacking amino acids 100 to 116 of the MbII domain (lane 3). Following transfection, cells were lysed as for panel A, immunoprecipitations were performed with anti-FLAG antibody; precipitates were resolved by SDS-PAGE and Western blotted for either the FLAG epitope (lower panel) or hGCN5 (upper panel). Mock transfected 293 cells served as a control (lane 1).