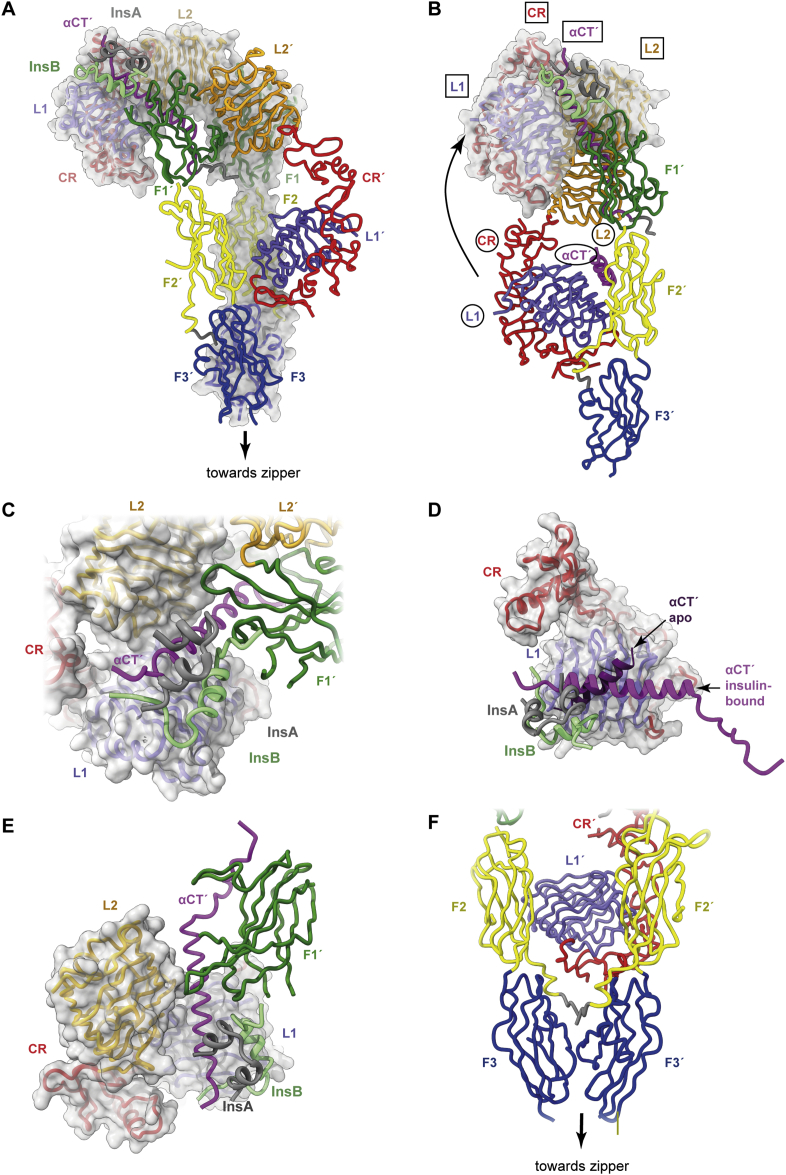
Figure 5.
Three-dimensional structure of the single-insulin-bound insulin receptor ectodomain. (A) Overall domain configuration of the single-insulin-bound insulin receptor ectodomain determined in the context of the leucine-zippered construct. (B) Schematic demonstrating how the L1, CR, and L2 domains and the αCT′ segment relocate to the receptor “head” upon insulin binding. Domain names are circled for the insulin-free elements and boxed for the insulin-bound elements. Only a single receptor “leg” is shown (that is, domains L1, CR, and L2 from one monomer and domains FnIII-1, FnIII-2, FnIII-3, and αCT′ from the alternate monomer). (C) Detail of (A) showing engagement of insulin with domain FnIII-1′. (D) Detail of (A) showing the reconfiguration of αCT′ upon the L1 surface upon insulin binding. (E) Detail of (A) showing engagement of domains L2 and FnIII-1′ with the extended αCT′ helix and engagement of domain L1 with domain L2 upon insulin binding. (F) Detail of (A) showing how the domains FnIII-2, FnIII-2, FnIII-3, and FnIII-3′ fold inwards with respect to the apo receptor ectodomain structure, forming an interaction between domains FnIII-3 and FnIII-3′ while retaining an apo-like association of domain L1′ with domain FnIII-2. Panels are based on PDB 6HN5 [13], PDB 6HN4 [13] and PDB 4ZXB [95].