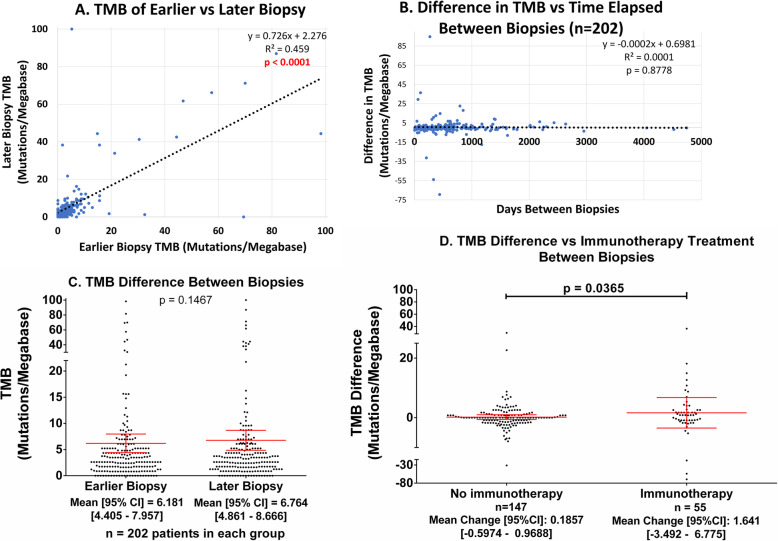
Fig. 1.
UCSD TMB differences. All patients with valid UCSD TMB data (n = 202) were considered for this figure. Exposure or treatment is defined as having received agent between the initial and final biopsies, regardless of dose or duration, as per electronic medical records. A The TMB of the earlier and later biopsies are somewhat correlated with a significant, almost unity, slope (p < 0.0001), and a Pearson R2 = 0.459. This indicates there is no difference between early and later TMBs. B TMB difference does not correlate with time elapsed between biopsies (Pearson correlation R2 = 0.0001). The slope of the line of best fit is also not significantly greater than zero (p = 0.8778). C There is no significant different between TMBs measured at different times as determined by the Wilcoxon matched pairs signed rank test. Red lines are mean (center) ± 95% confidence interval (CI) for each group. The average TMB of the earlier biopsy was 6.181 [4.405–7.957] mutations/Mb versus 6.764 [4.861–8.666] mutations/Mb for the later biopsy (p = 0.1467). D TMB difference (increase) with time in immunotherapy-treated patients is greater than in those who did not receive immunotherapy (immunotherapy response not considered). All patients with valid TMB data considered. Red lines are mean (center) ± 95% CI for each group. Patients who were treated with immunotherapy between biopsies had a mean ± 95% CI TMB change of 1.641 [− 3.492–6.775] mutations/Mb whereas those who were not had a mean ± 95% CI TMB change of 0.1857 [− 0.5974–0.9688] mutations/Mb (p = 0.0365). Drugs received included the following: ipilimumab, nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab, and cemiplimab