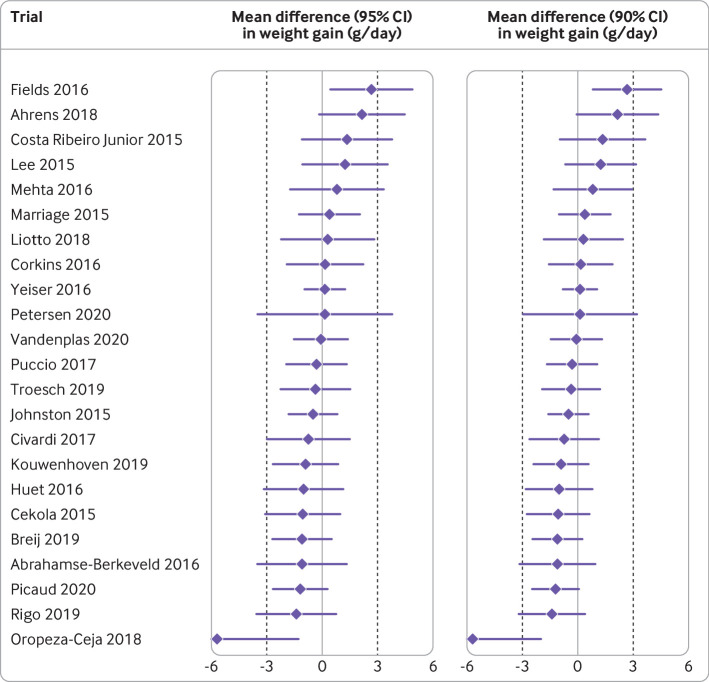
Fig 3.
Primary outcomes for formula trials designed to show adequate infant growth, published between 2015 and 2020. Data are mean differences (95% confidence intervals) and mean differences (90% confidence intervals) in weight gain between intervention and control formula during the intervention period, for the 23 trials that were designed according to guidance of Food and Drug Administration for demonstrating adequate growth. Equivalence margins of 3 g/day weight gain are indicated by dotted lines. Authors interpreted the primary outcome as favourable in 22 (96%) trials