Supplemental Digital Content is available in the text.
Key Words: Helicobacter pylori, modified dual therapy, first-line regimen, eradication, adverse effects
Background:
In an era of antibiotic resistance, modified dual therapy has been paid much attention because of simple drug composition and low resistance of amoxicillin. However, its eradication rate as a first-line regimen remains controversial. This study is to evaluate the efficacy and safety of modified dual therapy for the initial treatment of Helicobacter pylori (H. pylori) infection compared with mainstream first-line therapies.
Methods:
PubMed, the Cochrane Library, and Embase were searched for randomized clinical trials evaluating the efficacy and safety of modified dual therapy as the initial treatment for H. pylori eradication compared with guideline-recommended first-line therapies. A meta-analysis was conducted using Review Manager 5.3 and dichotomous data were estimated by the risk ratio (RR) with the 95% confidence interval (CI). We also performed subgroup analysis according to control groups and studies with antibiotic susceptibility tests.
Results:
Eight studies including 1672 patients with H. pylori infection met the selection criteria and were assessed. The meta-analysis demonstrated that modified dual therapy achieved similar efficacy [85.83% vs. 86.77%, RR 0.99 (95% CI, 0.95-1.03), intention-to-treat analysis; 89.53% vs. 90.45%, RR 0.99 (95% CI, 0.96-1.02), per-protocol analysis] and compliance [95.77% vs. 95.56%, RR 1.00 (95% CI, 0.98-1.02)] compared with recommended first-line regimens. In addition, there were no significant differences in comparing the eradication rate of modified dual therapy with clarithromycin triple therapy, bismuth quadruple therapy, and concomitant therapy, respectively. Subgroup analysis based on the studies with antibiotic susceptibility tests also confirmed a similar efficacy. However, modified dual therapy showed fewer adverse effects [8.70% vs. 22.38%, RR 0.39 (95% CI, 0.28-0.54)], with a significant difference (P<0.00001).
Conclusion:
Modified dual therapy achieved equal efficacy and compliance compared with recommended first-line regimens for H. pylori infection, and generally modified dual therapy showed fewer side effects.
Helicobacter pylori (H. pylori) is highly adapted for the colonization of the human stomach and has infected nearly half of the world’s population, including ~700 million individuals in China.1 H. pylori infection plays an important role in upper digestive diseases, such as gastritis, peptic ulcers, mucosa-associated lymphoid tissue lymphoma, and deadly gastric adenocarcinoma.2,3 Successful eradication of H. pylori could markedly improve the patient’s symptoms, attenuate the course of these diseases, and prevent gastric cancer.4
Traditional triple therapy consisting of clarithromycin, amoxicillin (or metronidazole), and a proton-pump inhibitor (PPI) has been used worldwide as the first-line regimen to eradicate H. pylori for decades and achieved eradication rates of >90% in the 1990s.5 However, due to increasing resistance to antibiotics especially clarithromycin, metronidazole, and levofloxacin,6,7 the success of clarithromycin triple therapy as well as several alternative regimens has rapidly declined.8,9 Toronto Consensus has opposed the use of sequential and hybrid therapies and did not recommend levofloxacin therapy as first-line therapies.10 At present, Maastricht V/Florence Consensus, Toronto Consensus, and American College of Gastroenterology Clinical Guideline still all recommended clarithromycin triple therapy as one of the first-line regimen, limited to areas where clarithromycin resistance is <15%. For areas of high clarithromycin resistance, bismuth quadruple therapy and concomitant therapy (when bismuth is not available such as Taiwan11) were favored.10,12,13 In China, resistance rates of clarithromycin range from 20% to 50%, therefore, bismuth quadruple therapy has been highly recommended as first-line therapy for H. pylori eradication according to the Fifth Chinese National Consensus Report.6,14 Despite the improvements in the eradication rate, quadruple therapy has limitations, including complex pharmaceutical composition, increasing side effects, and high cost.
In an era of high resistance, H. pylori resistance to amoxicillin has remained rare worldwide, including in China.6,7,15 Indeed, dual therapy consisting of amoxicillin (1 g, twice daily) and omeprazole (20 mg, once or twice daily) has been proposed since the 1980s.16 It was gradually forgotten and abandoned because of the unsatisfactory eradication rate. However, nowadays, the critical drug resistance remarkably limited the choice of antibiotics, and quadruple therapy could hardly be strengthened by adding new drugs or prolonging duration. Dual therapy has regained people’s attention. As is well known, amoxicillin is a pH-dependent and time-dependent antibiotic.17–19 Thus, primary dual therapy was modified by increasing both the dose and administration frequency of amoxicillin and PPI to improve the eradication rate. A meta-analysis showed that modified (or high-dose) dual therapy was comparable to recommended rescue therapies for the treatment of H. pylori infection.20 According to the American College of Gastroenterology Clinical Guideline, high-dose dual therapy consisting of amoxicillin and a PPI for 14 days has been suggested as a salvage regimen.12
An increasing number of studies have been conducted to investigate the efficacy and safety of modified dual therapy as an initial treatment for H. pylori infection. A small-sample multicenter clinical study in Italy showed that high-dose dual therapy with esomeprazole and amoxicillin might be effective and safe as a first-line regimen.21 However, modified dual therapy has not yet been recommended as a first-line regimen, mainly because of the conflicting eradication rates among different studies. An open-labeled study showed that high-dose amoxicillin-PPI dual therapy was ineffective as the first-line therapy for eradicating H. pylori in Korea.22 Hu and colleagues also reported that high-dose amoxicillin-PPI dual therapy failed to achieve high cure rates in China.23 Therefore, we conducted a meta-analysis to investigate the efficacy and safety of modified dual therapy as primary therapy for H. pylori infection, compared with mainstream first-line regimens, including traditional clarithromycin triple therapy, bismuth quadruple therapy and nonbismuth quadruple therapy (concomitant therapy).
METHODS
Search Strategy
This meta-analysis was conducted according to the reporting guidelines of PRISMA protocol.24 We searched PubMed, the Cochrane Library, and Embase for studies published up to February 26, 2020, without language and publication status restriction. The following terms and algorithms were used as retrieval strategies. PubMed: [“Helicobacter pylori” (Mesh) OR H. pylori] AND (dual therapy) AND [“randomized controlled trial” (Publication type) OR randomized]; the Cochrane Library: [Helicobacter pylori (Mesh) OR H. pylori] AND (dual therapy); Embase: (“Helicobacter pylori”/exp OR H. pylori) AND (dual therapy) AND (“randomized controlled trial”/exp). We also manually checked the reference lists of systematic reviews, comments, and included randomized controlled trials (RCTs) to include other potentially qualified studies.
Selection Criteria
Endnote X9 (Thomson Reuters, Philadelphia, PA) was used to create an electronic library of articles identified in the literature retrieval. Literature was then screened according to the selection criteria. Inclusion criteria were as follows: (a) studies evaluated the efficacy and safety of modified dual therapy as the first-line regimen to eradicate H. pylori; (b) the control groups included clarithromycin triple therapy, bismuth quadruple therapy, and concomitant therapy; (c) the study design was RCT. Exclusion criteria were set as below: (a) studies on animals, case reports or case series, retrospective study, cohort study, and studies without controls or the control group was not appropriate; (b) studies not reporting tests used to diagnose H. pylori infection and eradication; (c) regimen contained herbs, probiotics, or other supplements; (d) amoxicillin was not administered at a dosage >2.0 g daily and either amoxicillin or PPI was given no more than twice daily. According to current clinical guidelines, the duration of treatment with clarithromycin triple therapy has been increased from 7 to 14 days. Therefore, Yang et al’s study who used clarithromycin triple therapy for 7 days as a control group was excluded.11
Data Extraction
Two authors (Q.H. and H.Y.) extracted data independently, and disagreement was resolved by discussion with the third author (Z.S.). The following information was extracted: (a) the first author, year of publication, country or region, and patients’ demographic characteristics; (b) tests used to confirm H. pylori infection and eradication; (c) therapeutic regimens, duration of treatment, number of patients in each group; (d) the results of antibiotics susceptibility tests and Cytochrome P450 (CYP) 2C19 genotype; (e) eradication rate by intention-to-treat (ITT) and per-protocol analyses, the compliance of patients and number of patients with side effects as defined within each included RCTs.
Outcome Assessment
The primary outcome for the meta-analysis was overall eradication rates of modified dual therapy compared with established first-line therapies. And we compared the eradication rates of modified dual therapy with clarithromycin triple therapy, bismuth quadruple therapy, and concomitant therapy, respectively. To avoid interference of antibiotic resistance on the control groups, a subgroup analysis of studies with antibiotic susceptibility tests was conducted. The secondary outcomes were the incidence of adverse effects and compliance of patients in modified dual therapy versus other first-line therapies.
Risk of Bias and Sensitivity Analysis
The risk of bias of the included studies was assessed using the domain-based risk of bias tables. Two authors (Q.H. and H.Y.) evaluated the risk of bias independently. Any disagreement was resolved by consensus. A funnel plot was constructed to evaluate the risk of publication bias. We also performed a sensitivity analysis to test the stability of the results.
Statistical Analysis
Meta-analysis was conducted by Review Manager 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark). Dichotomous outcomes were determined using the Mantel-Haenszel method and summarized as the risk ratio (RR) with the 95% confidence interval (CI). P<0.05 was considered a significant difference. The heterogeneity of the included studies was evaluated by the inconsistency index (I 2) statistic and the χ2 test. I 2>50% or P<0.1 for the χ2 test indicated statistical heterogeneity.25 If there was significant heterogeneity in included RCTs, we used the random-effects model. When no significant heterogeneity was found, the fixed-effects model was selected to pool the data.
RESULTS
Characteristics of the Selected Studies
A total of 390 studies were obtained during the literature search, of which 202 studies were excluded because of duplicates. After abstract retrieval, 116 studies were excluded because they were irrelevant to the current analysis or not RCTs. A total of 72 articles were retrieved by reading the full text, and 22 were further excluded because modified dual therapy was not a primary treatment or the control group was not appropriate. Then, 42 studies were excluded because either amoxicillin or PPI was given less than thrice daily, or amoxicillin was administrated at no more than 2.0 g/d in the modified dual therapy. Finally, 8 prospective RCTs including 1672 participants (833 with modified dual therapy and 839 with the control therapy) met the selection criteria and there was no publication bias (Fig. S1, Supplemental Digital Content 1, http://links.lww.com/JCG/A616). The flowchart of the study selection was displayed in Figure 1, and the characteristics of the 8 included RCTs were summarized in Table 1 and Table S1 (Supplemental Digital Content 1, http://links.lww.com/JCG/A616).
FIGURE 1.
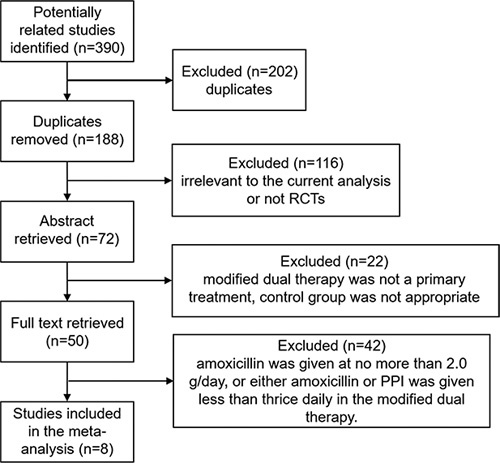
Flowchart of literature selection. PPI indicates proton pump inhibitor; RCTs, randomized controlled trials.
TABLE 1.
Study Characteristics
| References | Initial Diagnosis/Re-diagnosis | Subgroup (n/N) | Regimens | Eradication Rate, % (ITT/PP) |
|---|---|---|---|---|
| Kim et al26 | RUT or histology/RUT, histology and 13C-UBT | MDT (88/104) | AMO 750 mg tid, LAN 30 mg tid 14 d | 67.3/78.4 |
| Control (93/104) | AMO 1 g bid, CLA 500 mg bid, LAN 30 mg bid 14 d | 74.0/82.8 | ||
| Hu et al27 | Histology and culture/13C-UBT | MDT (167/170) | AMO 750 mg qid, RAB 20 mg qid 14 d | 94.7/96.4 |
| Control (164/170) | RAB 20 mg bid, TDB 300 mg qid, MTZ 250 mg qid, TET 500 mg qid 10 d | 90.6/93.3 | ||
| Hu et al23 | 13C-UBT, RUT and culture/13C-UBT | MDT (86/87) | AMO 750 mg qid, RAB 10 mg qid 14 d | 78.1/79.1 |
| Control (87/89) | AMO 1 g bid, CLA 500 mg bid RAB 20 mg bid B 220 mg bid 14 d | 84.3/86.2 | ||
| Sapmaz et al28 | Histology/stool H. pylori antigen test | MDT (93/98) | AMO 750 mg qid, RAB 20 mg tid 14 d | 84.7/84.9 |
| Control (89/98) | RAB 20 mg bid, BS 120 mg qid, TET500 mg qid, MTZ 500 mg tid 14 d | 87.8/88.8 | ||
| Gao et al29 | 13C or 14C-UBT,histology/13C or 14C-UBT | MDT (65/70) | AMO 750 mg qid, ESO 20 mg qid 14 d | 82.9/89.2 |
| Control (66/72) | AMO 1 g bid, CLA 500 mg bid, ESO 20 mg bid, BPC 220 mg bid 14 d | 86.1/93.9 | ||
| Leow et al30 | RUT/13C-UBT | MDT (67/68) | AMO 1 g qid, RAB 20 mg qid 14 d | 92.7/94.0 |
| Control (69/70) | AMO 1 g bid, RAB 20 mg bid, CLA 500 mg bid 14 d | 92.9/94.2 | ||
| Tai et al31 | Culture and RUT /13C-UBT | MDT (115/120) | AMO 750 mg qid, ESO 40 mg tid 14 d | 91.7/95.7 |
| Control (114/120) | AMO 1 g bid, CLA 500 mg bid, ESO 40 mg bid, MTZ 500 mg bid 7 d | 87.5/92.1 | ||
| Yang et al32 | 13C-UBT and RUT/13C-UBT | MDT (112/116) | AMO 750 mg qid, ESO 20 mg qid 14 d | 87.9/91.1 |
| Control (114/116) | AMO 1 g bid, CLA 500 mg bid, ESO 20 mg bid BPC 1 g (containing 220 mg B) bid 14 d | 89.7/91.2 |
AMO indicates amoxicillin; B, bismuth; bid, twice daily; BPC, bismuth potassium citrate; BS, bismuth subcitrate; CLA, clarithromycin; ESO, esomeprazole; H. pylori, Helicobacter pylori; ITT, intention-to-treat; LAN, lansoprazole; MDT, modified dual therapy; MTZ, metronidazole; PP, per-protocol; qid, four times daily; RAB, rabeprazole; RUT, rapid urease test; TDB, tripotassium dicitrate bismuthate; TET, tetracycline; tid, three times daily; UBT, urea breath test.
Overall Eradication Rate
The eradication rates of H. pylori were evaluated in all 8 studies. No significant difference was observed between modified dual therapy and the control therapies. In terms of ITT analysis, the pooled eradication rate was 85.83% for the modified dual therapy compared with 86.77% for the control therapies [RR=0.99, 95% CI (0.95-1.03), P=0.57], without significant statistical heterogeneity (P=0.43, I 2=0%, Fig. 2). As for per-protocol analysis, the pooled RR was 0.99 [95% CI (0.96-1.02), P=0.51], with no evidence of heterogeneity (P=0.38; I 2=6%, Fig. 3). The overall eradication rate was 89.53% for the modified dual therapy compared with 90.45% for the control groups.
FIGURE 2.
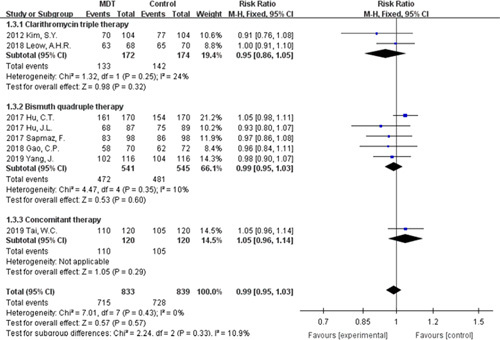
Forest plot of Helicobacter pylori eradication rate (intention-to-treat analysis). CI indicates confidence interval; MDT, modified dual therapy.
FIGURE 3.
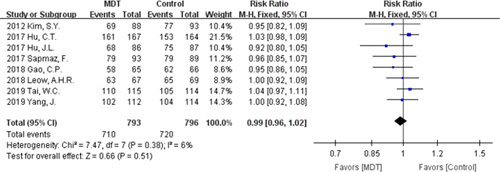
Forest plot of Helicobacter pylori eradication rate (per-protocol analysis). CI indicates confidence interval; MDT, modified dual therapy.
Subgroup Analysis According to Control Groups
Control groups differed among the 8 RCTs, namely clarithromycin triple therapy recommended by Kim et al26 and Leow and Goh,30 bismuth quadruple therapy recommended by Hu et al,27 Hu et al,23 Sapmaz et al,28 Gao et al,29 and Yang et al,32 and concomitant therapy recommended by Tai et al.31 Whether it is compared with clarithromycin triple therapy, bismuth quadruple therapy, or the concomitant therapy, the modified dual therapy has achieved a similar eradication rate without significant statistical heterogeneity [RR=0.95, 95% CI (0.86-1.05); RR=0.99, 95% CI (0.95-1.03), RR=1.05, 95% CI (0.96-1.14); respectively, Fig. 2]. Clarithromycin was contained in most of the regimens, including all clarithromycin triple regimens, concomitant therapy, and bismuth quadruple therapy in some RCTs. To exclude the influence of clarithromycin on the result, we removed regimens containing clarithromycin and there were 2 studies remaining. The subgroup analysis showed that the efficacy of modified dual therapy was comparable [91.04% vs. 89.55%, RR=1.02, 95% CI (0.96-1.07), P=0.56] to the control therapies, without significant statistical heterogeneity (P=0.2, I 2=40%; Fig. S2, Supplemental Digital Content 1, http://links.lww.com/JCG/A616).
Subgroup Analysis According to Studies With Antibiotic Susceptibility Tests
Three studies reported the results of antibiotic susceptibility test (Table S2, Supplemental Digital Content 1, http://links.lww.com/JCG/A616). The subgroup analysis also demonstrated that modified dual therapy achieved similar efficacy [91.87% vs. 89.41%, RR=1.03, 95% CI (0.98-1.07), P=0.23] compared with mainstream first-line therapies, without significant statistical heterogeneity (P=0.47, I 2=0%; Fig. 4).
FIGURE 4.

Subgroup analysis of Helicobacter pylori eradication rate according to studies with antibiotic susceptibility tests. CI indicates confidence interval; MDT, modified dual therapy.
Compliance and Adverse Effects
Both therapies achieved a high compliance rate, with 95.77% for the modified dual therapy and 95.56% for the control therapies with no statistical heterogeneity (P=0.75; I 2=0%, Fig. 5). And there was no significant statistical difference [RR=1.00, 95% CI (0.98-1.02), P=0.83]. Five studies reported the specific number of patients with adverse effects in the treatment. The overall adverse effects rate was 8.70% for the modified dual therapy and 22.38% for the control therapies, and we found no statistical heterogeneity [P=0.56; I 2=0%, Fig. 6]. Notably, modified dual therapy was associated with a significantly decreased risk of adverse effects [RR=0.39, 95% CI (0.28-0.54), P<0.00001].
FIGURE 5.
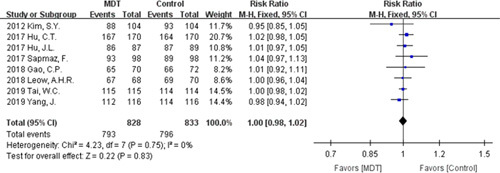
Forest plot of compliance. CI indicates confidence interval; MDT, modified dual therapy.
FIGURE 6.
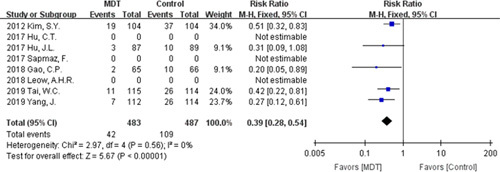
Forest plot of adverse effects. CI indicates confidence interval; MDT, modified dual therapy.
Risk of Bias and Sensitivity Analysis
We assessed the potential bias of each study through several aspects, including selection bias, performance bias, detection bias, attrition bias, and reporting bias. The risk of bias was shown in Figure 7. The sensitivity analysis did not change either the direction or the statistical significance of any of the RRs.
FIGURE 7.
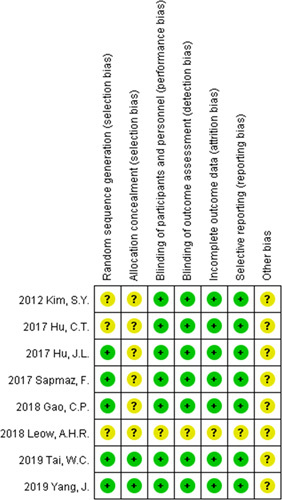
Risk of bias summary.
DISCUSSION
H. pylori’s eradication is facing a critical challenge due to antibiotic resistance. Guidelines and experts recommend that regimens based on antibiotic susceptibility test results are optimal; however, due to the invasiveness of endoscopy, the difficulty of H. pylori’s culture, and cost concerns, antibiotic susceptibility test is not performed routinely in clinical practice. Fortunately, the global prevalence of primary and acquired H. pylori resistance to amoxicillin is still generally rare.7 Therefore, amoxicillin can be used empirically without the need for susceptibility testing. Both triple therapy and quadruple therapy require the continuous use of 2 or 3 antibiotics, which increases the patient’s antibiotic load and drug cost, leading to more side effects.33 Once eradication failed, antibiotic choices become trickier. Modified dual therapy, which uses only 1 antibiotic, amoxicillin, has the advantage of reducing antibiotic use, reducing cost, and providing room for eradication failure. In addition, elderly patients and patients with multiple complications cannot afford such a complex treatment.34 A regimen with fewer medications was demanded especially for them. Therefore, it is of great importance to identify the efficacy and safety of modified dual therapy as the first-line regimen. We conducted this meta-analysis and the results showed that modified dual therapy was equally effective as the recommended first-line regimens for H. pylori infection and safer than those therapies.
However, from the perspective of eradication rate, modified dual therapy was not superior to the currently recommended first-line therapies, and few studies with eradication rate >90% (the definition of eradication success by Graham et al35) have been done. Studies with eradication rates above 90% pointed the key to success: the concentration of amoxicillin and proper intragastric acid environment. As is known, amoxicillin is time-dependent penicillin, and plasma levels over the minimum inhibitory concentrations could be maintained for 6 to 8 hours; thus, plasma concentrations of amoxicillin cannot be achieved by the traditional administration of 1 g twice daily.19 Increasing the dosage and administration frequency of amoxicillin simultaneously could maximize the pharmacokinetic and pharmacodynamic effects and achieve a satisfactory eradication rate.36 The eradication rate of Kim’s study (750 mg amoxicillin 3 times daily) was hardly satisfactory and lower than that of other studies (750 mg amoxicillin 4 times daily), showing that the latter regimen was a better choice. Lu and colleague’s study conducted in Shanghai also achieved a satisfactory eradication rate.37 They used 1 g amoxicillin 3 times daily, which is an alternative method of administration.
The intragastric pH value is mainly associated with the type, dosage, and administration frequency of PPIs. Second-generation PPIs (esomeprazole, rabeprazole) may be more effective than first-generation PPIs (omeprazole, lansoprazole) because they have been demonstrated to be less dependent on the genetic variability of CYP2C19.38,39 Ren and colleagues compared the eradication rate of an R10A (amoxicillin 1 g thrice daily plus rabeprazole 10 mg twice daily) group and an R20A (amoxicillin 1 g thrice daily plus rabeprazole 20 mg twice daily) group. The results showed that the eradication rate reached 89.8% in the R20A group by ITT analysis, which was significantly higher than that reached in the R10A group.36 Sugimoto and colleagues compared the effects of different frequencies of administration at the same total daily dose. The results demonstrated that increasing the dosing frequency of rabeprazole effectively increased the pH value.40 The appropriate dosage and administration frequency of various PPIs should be tested to achieve optimal intragastric pH. Besides, vonoprazan is a novel potassium-competitive acid blocker which is more potent and long-acting than traditional PPIs.41–43 Pharmacokinetics demonstrated that vonoprazan was mainly metabolized by CYP3A4, accordingly less affected by CYP2C19 polymorphism.44 Furuta et al’s45 study showed that vonoprazan-based dual therapy with amoxicillin is effective and safe for the eradication of H. pylori. The efficacy of amoxicillin-vonoprazan dual therapy is worth further study.46,47 However, there were no suitable RCTs comparing the efficacy of amoxicillin-vonoprazan dual therapy with mainstream first-line therapies, therefore vonoprazan-amoxicillin dual therapy was not included.
Other factors including dietary management and smoking also affected gastric acid environment. In Yang et al’s11 study, who reported a high eradication rate, patients were instructed to avoid acidic foods to minimize the impact of ingested foods to intragastric acidity. According to the latest study by Lu and colleagues, smokers had a markedly reduced eradication rate (7/10) compared with nonsmokers (66/66) with high-dose amoxicillin-esomeprazole dual therapy.37 Same therapy plus bismuth could only improve treatment effectiveness among smokers. In addition, compliance is also a key factor for the eradication rate, especially when the regimen contains multiple medications that need to be taken several times. Studies have found that improved compliance through enhanced visits and patient education could effectively improve eradication rate.48,49 The new drug Talicia has inspired us to develop amoxicillin and PPI combined sustained-release capsules that may improve compliance and efficacy, which remains to be studied. In a word, how to improve the eradication rate of H. pylori is the challenge we are facing.
In terms of adverse effects, modified dual therapy was superior to the recommended first-line therapies. Overall, modified dual therapy reported no serious adverse reactions. The main adverse events included nausea, abdominal pain, diarrhea, and rash. Most adverse effects disappeared spontaneously after treatment. The result is reasonable: modified dual therapy uses only 1 antibiotic and does not contain bismuth, resulting in lower overall side effects. As for compliance, modified dual therapy was comparable to mainstream first-line therapies. Although the modified dual therapy increased the dosage and dosing frequency of amoxicillin and PPI, it reduced the number of medications and has fewer side effects, leading to high compliance.
There are some limitations in our meta-analysis. First, the participants enrolled in RCTs are mainly from Asia, typically China. Therefore, further research needs to be conducted among people of different populations to avoid interference from ethnic differences in PPI metabolism. Second, most studies were not blinded, which may lead to bias in reporting side effects. Indeed, blindness is not necessary for the primary outcome, eradication rate. Last, due to the lack of included studies and related reports (Table S3, Supplemental Digital Content 1, http://links.lww.com/JCG/A616), subgroup analysis according to CYP2C19 genotype was not possible. However, Yang’s study showed that high dose and administration frequency of esomeprazole ameliorates the influence of CYP2C19 genotype and there was no significant correlation between the treatment outcomes and CYP2C19 genotype.32 It means modified dual therapy (proper PPI) could be effective regardless of CYP2C19 genotype and be used widely.
In conclusion, our meta-analysis showed that modified dual therapy could achieve similar eradication rates and compliance compared with recommended first-line therapies as well as lower side effects. Since most of the RCTs were conducted in China and there is an increasing resistance to clarithromycin and metronidazole, the result may have a great significance for China to optimize regimens. More high-quality RCTs are needed to confirm the hypothesis that modified dual therapy could be an alternative first-line regimen for the treatment of H. pylori infection.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.
Footnotes
Q.H. and H.Y.: searched the literature, collected data, analyzed and interpreted data, and drafted articles. Z.S.: participated in the acquisition of data. H.C.: participated in analyzing data and writing the article. X.Z.: reviewed and approved the publication of the article.
Supported by National Natural Science Foundation of China, No. 81973615
The authors declare that they have nothing to disclose.
REFERENCES
- 1.Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868–876. [DOI] [PubMed] [Google Scholar]
- 2.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018;378:1085–1095. [DOI] [PubMed] [Google Scholar]
- 5.European Helicobacter Pylori Study Group. Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. European Helicobacter Pylori Study Group. Gut. 1997;41:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y, Zhu Y, Lu NH. Primary antibiotic resistance of Helicobacter pylori in China. Dig Dis Sci. 2017;62:1146–1154. [DOI] [PubMed] [Google Scholar]
- 7.Savoldi A, Carrara E, Graham DY, et al. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155:1372–1382.e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang BN, Graham DY. Helicobacter pylori infection and antibiotic resistance: a WHO high priority? Nat Rev Gastroenterol Hepatol. 2017;14:383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B, Lv ZF, Wang YH, et al. Standard triple therapy for Helicobacter pylori infection in China: a meta-analysis. World J Gastroenterol. 2014;20:14973–14985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallone CA, Chiba N, van Zanten SV, et al. The Toronto Consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151:51–69.e14. [DOI] [PubMed] [Google Scholar]
- 11.Yang JC, Lin CJ, Wang HL, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2015;13:895–905.e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chey WD, Leontiadis GI, Howden CW, et al. ACG Clinical Guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239. [DOI] [PubMed] [Google Scholar]
- 13.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- 14.Liu WZ, Xie Y, Lu H, et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. [DOI] [PubMed] [Google Scholar]
- 15.Kuo YT, Liou JM, El-Omar EM, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:707–715. [DOI] [PubMed] [Google Scholar]
- 16.Unge P, Gad A, Gnarpe H, et al. Does omeprazole improve antimicrobial therapy directed towards gastric Campylobacter pylori in patients with antral gastritis?: a pilot study. Scand J Gastroenterol. 1989;167:49–54. [DOI] [PubMed] [Google Scholar]
- 17.Scott D, Weeks D, Melchers K, et al. The life and death of Helicobacter pylori. Gut. 1998;43(suppl 1):S56–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. [DOI] [PubMed] [Google Scholar]
- 19.Berry V, Jennings K, Woodnutt G. Bactericidal and morphological effects of amoxicillin on Helicobacter pylori. Antimicrob Agents Chemother. 1995;39:1859–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao CP, Zhou Z, Wang JZ, et al. Efficacy and safety of high-dose dual therapy for Helicobacter pylori rescue therapy: a systematic review and meta-analysis. J Dig Dis. 2016;17:811–819. [DOI] [PubMed] [Google Scholar]
- 21.Zullo A, Ridola L, Francesco VD, et al. High-dose esomeprazole and amoxicillin dual therapy for first-line Helicobacter pylori eradication: a proof of concept study. Ann Gastroenterol. 2015;28:448–451. [PMC free article] [PubMed] [Google Scholar]
- 22.Kwack W, Lim Y, Lim C, et al. High dose ilaprazole/amoxicillin as first-Line regimen for Helicobacter pylori infection in Korea. Gastroenterol Res Pract. 2016;2016:1648047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu JL, Yang J, Zhou YB, et al. Optimized high-dose amoxicillin–proton-pump inhibitor dual therapies fail to achieve high cure rates in China. Saudi J Gastroenterol. 2017;23:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SY, Jung SW, Kim JH, et al. Effectiveness of three times daily lansoprazole/amoxicillin dual therapy for Helicobacter pylori infection in Korea. Br J Clin Pharmacol. 2012;73:140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu CT, Tung CC, Lin CJ, et al. Efficacy of high-dose dual therapy versus bismuthcontaining quadruple therapy for first-line treatment of helicobacter pylori infection and an interim report of multi-center, randomized control study. Gastroenterology. 2017;152:S182–S183. [Google Scholar]
- 28.Sapmaz F, Kalkan IH, Atasoy P, et al. A non-inferiority study: modified dual therapy consisting higher doses of rabeprazole is as successful as standard quadruple therapy in eradication of Helicobacter pylori. Am J Therap. 2017;24:e393–e398. [DOI] [PubMed] [Google Scholar]
- 29.Gao CP, Xiao X, Liu PX, et al. High-dose amoxicillin/esomeprazole dual therapy as a first-line therapy for Helicobacter pylori eradication. World Chinese J Digestol. 2018;26:353–359. [Google Scholar]
- 30.Leow AHR, Goh KL. 14-day high-dose dual therapy is equally as good as 14-day clarithromycin based standard triple therapy in first line H. pylori eradication treatment. J Gastroenterol Hepatol. 2018;33:363. [Google Scholar]
- 31.Tai WC, Liang CM, Kuo CM, et al. A 14 day esomeprazole- and amoxicillin-containing high-dose dual therapy regimen achieves a high eradication rate as first-line anti-Helicobacter pylori treatment in Taiwan: a prospective randomized trial. J Antimicrob Chemother. 2019;74:1718–1724. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Zhang Y, Fan L, et al. Eradication efficacy of modified dual therapy compared with bismuth-containing quadruple therapy as a first-line treatment of Helicobacter pylori. Am J Gastroenterol. 2019;114:437–445. [DOI] [PubMed] [Google Scholar]
- 33.Hsu PI, Pan CY, Kao JY, et al. Helicobacter pylori eradication with bismuth quadruple therapy leads to dysbiosis of gut microbiota with an increased relative abundance of Proteobacteria and decreased relative abundances of Bacteroidetes and Actinobacteria. Helicobacter. 2018;23:e12498. [DOI] [PubMed] [Google Scholar]
- 34.Gao W, Ye H, Deng X, et al. Rabeprazole-amoxicillin dual therapy as first-line treatment for H pylori eradication in special patients: a retrospective, real-life study. Helicobacter. 2020;25:e12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177–186.e3; discussion e12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren L, Lu H, Li HY, et al. New dual therapy for primary treatment of Helicobacter pylori infection: a prospective randomized study in Shanghai, China. J Dig Dis. 2014;15:622–627. [DOI] [PubMed] [Google Scholar]
- 37.Yu L, Luo L, Long X, et al. High-dose PPI-amoxicillin dual therapy with or without bismuth for first-line Helicobacter pylori therapy: a randomized trial. Helicobacter. 2019;24:e12596. [DOI] [PubMed] [Google Scholar]
- 38.McNicholl AG, Linares PM, Nyssen OP, et al. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;36:414–425. [DOI] [PubMed] [Google Scholar]
- 39.Ierardi E, Losurdo G, Fortezza RF, et al. Optimizing proton pump inhibitors in Helicobacter pylori treatment: old and new tricks to improve effectiveness. World J Gastroenterol. 2019;25:5097–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugimoto M, Shirai N, Nishino M, et al. Rabeprazole 10 mg q.d.s. decreases 24-h intragastric acidity significantly more than rabeprazole 20 mg b.d. or 40 mg o.m., overcoming CYP2C19 genotype. Aliment Pharmacol Ther. 2012;36:627–634. [DOI] [PubMed] [Google Scholar]
- 41.Oshima T, Miwa H. Potent potassium-competitive acid blockers: a new era for the treatment of acid-related diseases. J Neurogastroenterol Motil. 2018;24:334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham DY, Dore MP. Update on the use of vonoprazan: a competitive acid blocker. Gastroenterology. 2018;154:462–466. [DOI] [PubMed] [Google Scholar]
- 43.Hunt RH, Scarpignato C. Potent acid suppression with PPIs and P-CABs: what’s new? Curr Treat Options Gastroenterol. 2018;16:570–590. [DOI] [PubMed] [Google Scholar]
- 44.Yamasaki H, Kawaguchi N, Nonaka M, et al. In vitro metabolism of TAK-438, vonoprazan fumarate, a novel potassium-competitive acid blocker. Xenobiotica. 2017;47:1027–1034. [DOI] [PubMed] [Google Scholar]
- 45.Furuta T, Yamade M, Kagami T, et al. Dual therapy with vonoprazan and amoxicillin is as effective as triple therapy with vonoprazan, amoxicillin and clarithromycin for eradication of Helicobacter pylori. Digestion. 2019:1–9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki S, Gotoda T, Shibuya H, et al. Efficacy of 7-day vonoprazan and amoxicillin dual therapy as firstline helicobacter pylori treatment: protocol of multi-center, noninferiority, randomized control trial. Gut. 2019;68:A87–A88. [Google Scholar]
- 47.Suzuki S, Gotoda T, Kusano C, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020;69:1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang T, Yang X, Li Y, et al. Twice daily short-message-based re-education could improve Helicobacter pylori eradication rate in young population: a prospective randomized controlled study. Helicobacter. 2019;24:e12569. [DOI] [PubMed] [Google Scholar]
- 49.Al-Eidan FA, McElnay JC, Scott MG, et al. Management of Helicobacter pylori eradication--the influence of structured counselling and follow-up. Br J Clin Pharmacol. 2002;53:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.


