Figure 1.
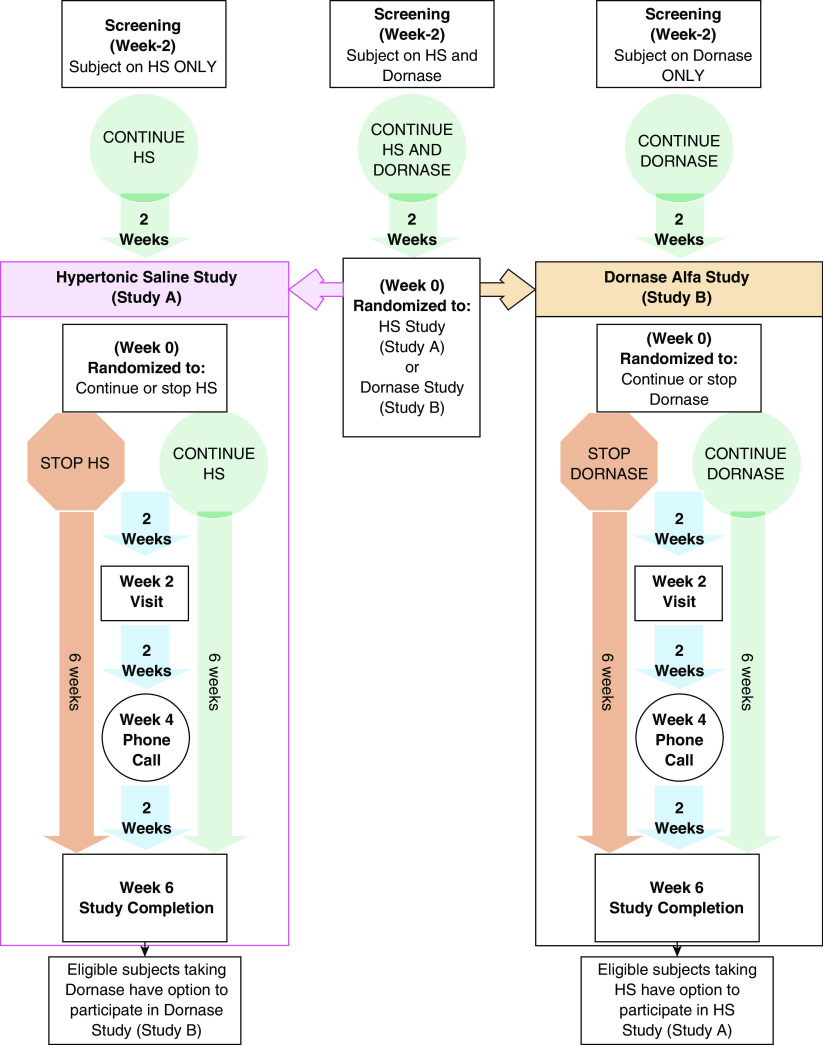
SIMPLIFY study design schematic. Study A and study B are identical randomized, open-label, two-arm trials consisting of a 2-week screening period and randomization to either continue or discontinue hypertonic saline (HS) (study A) or dornase alfa (Dornase) (study B), followed by a 6-week study period. Study visits occur at Weeks −2 (screening), 0, 2, and 6. Only those who maintain adequate reported adherence to inhaled drug therapy between screening (Week −2) and Week 0 are eligible for randomization (Table 1). At Week 0, subjects currently being treated with only HS or Dornase will be enrolled in study A or study B (as applicable) and will be randomized 1:1 to either continue or discontinue their current prescribed therapy. At study entry, subjects who are currently being treated with both HS and Dornase will remain on both therapies during the screening period and then be randomized to study A (HS) or study B (Dornase) as well as being randomized (1:1) to continue versus discontinue the applicable therapy. The randomization to study A or study B among subjects on both therapies is not optional and is essential to reduce indication bias and ensure comparable populations across studies. After completion of the first study, these subjects may subsequently enroll in the alternative study if they meet eligibility criteria. Reenrolling subjects need not remain on the treatment regimen assigned in the first study but must meet all eligibility criteria regarding treatment stability before entry (Table 1). Within each study, randomization will be stratified by the Week 0 percent-predicted forced expiratory volume in 1 second (⩾90, <90), treatment combination at screening (single or concurrent use of HS and/or Dornase), prior study participation (yes/no), and age (⩾18 versus <18). For subjects randomly assigned to continue their therapy during a given study, this therapy is expected to be taken at least once daily according to each subject’s preexisting, clinically prescribed regimen. If one study completes enrollment faster than the other study, the protocol will be restricted to enrollment in only the open study.