Figure 1.
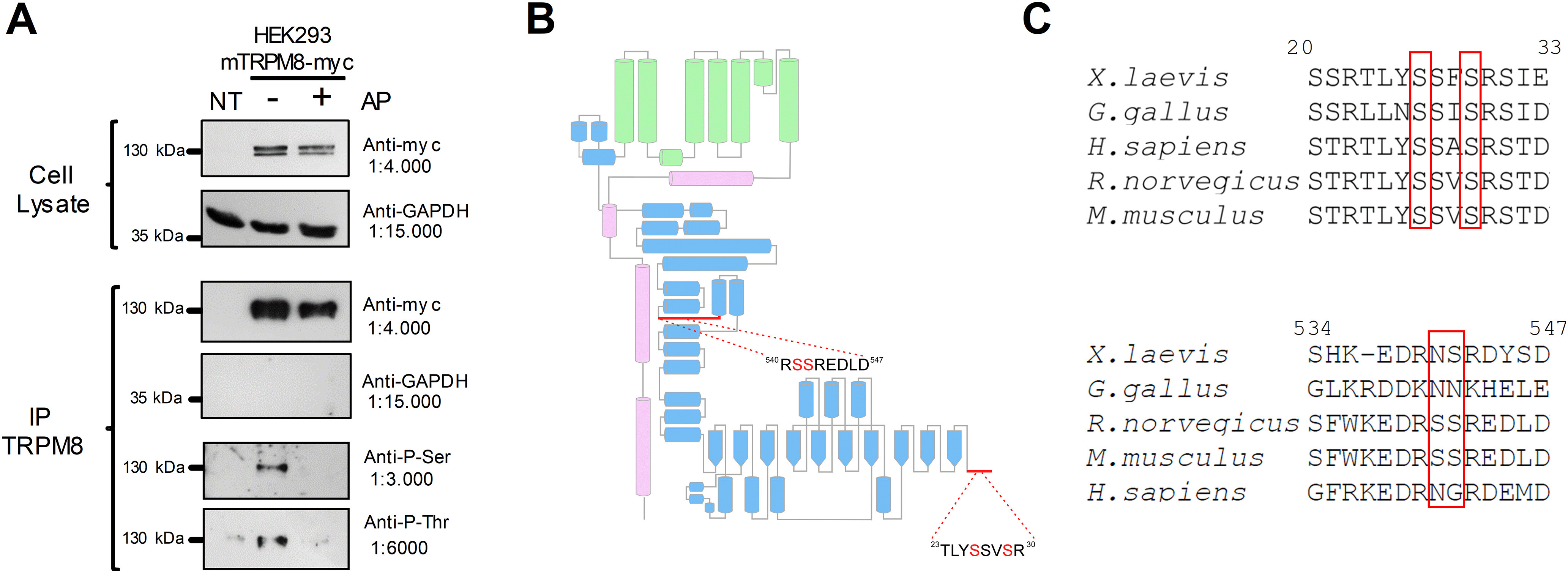
The TRPM8 channel is a phosphoprotein in basal conditions. A, Immunopurification (IP) of TRPM8 with anti-myc antibody from nontransfected HEK293 cells (NT) and HEK293-mTRPM8-myc cells extracts, untreated or treated with alkaline phosphatase (AP). Western blot of cell extracts and IPs showing the bands corresponding to TRPM8-myc and GAPDH. Phosphorylation of Ser and Thr residues were determined using phosphoserine (Anti-P-Ser) and phosphothreonine (Anti-P-Thr) antibodies. B, Scheme of a TRPM8 subunit showing its secondary structure elements described by Yin et al. (2018). Green represents transmembrane domains. Blue represents N-terminal domain. Magenta represents C-terminus. Modified from Pertusa et al. (2018). Close-up view of the proximal and distal N-terminal domain, showing the detail of the sequence where phosphorylated residues were identified using MS. C, Alignment of distal and proximal N-terminal domains of Xenopus laevis, Gallus gallus, Rattus norvegicus, Mus musculus, and Homo sapiens TRPM8 orthologs using ClustalW (Sievers et al., 2011) and Jalview (Waterhouse et al., 2009). Numbers correspond to residues in mTRPM8. Red boxes highlight the conservation of phosphorylation sites.
